Article Text
Abstract
Background The use of tranexamic acid (TXA) has become increasingly prevalent for hemorrhage prevention in military trauma patients due to its known survival benefits. There is concern of increased venous thromboembolism (VTE) subsequent to receiving TXA. The purpose of this retrospective study was to determine the rate of VTE in severely injured military personnel during Operation Enduring Freedom (2009–2014).
Methods An analysis of 859 military trauma patients from the 2009–2014 Department of Defense Trauma Registry included subjects with an injury severity score (ISS) >10 and a massive transfusion (MT) (>10 units of blood products in the first 24 hours). Outcomes included a documented VTE (eg, deep vein thrombosis (DVT) or pulmonary embolism (PE)) during the patient’s hospital course. Comparison between those who did/did not receive TXA was analyzed using three separate multiple regression analyses using listwise deletion, systematic replacement and multiple imputation.
Results Subjects (n=620) met inclusion criteria with 27% (n=169) having a documented VTE. A total of 30% that received TXA had a documented VTE, 26% that did not receive TXA had a documented VTE and 43% (n=264, n=620) of the sample did not have TXA documented as either given or not given. Multiple regression analyses using listwise deletion and systematic replacement of the TXA variable demonstrated no difference in odds of VTE, whereas the multiple imputation analysis demonstrated a 3% increased odds of VTE, a9.4% increased odds of PE and 8.1% decreased odds of DVT with TXA administration.
Discussion TXA use with an ISS >10 and MT resuscitation had a 3% increased odds of VTE and an increased odds of PE, whereas the odds of DVT were found to be decreased after multiple imputation analysis. Further research on the long-term risks and benefits of TXA usage in the military population is recommended.
Level of evidence IV—therapeutic.
- trauma
- surgery
- tranexamic acid
- pulmonary embolism
- deep vein thrombosis
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Background
In trauma patients, uncontrolled post-traumatic hemorrhage accounts for a significant percentage of mortality.1–3 When the body’s natural blood coagulation mechanisms malfunction and cause an inappropriate increase in blood clot breakdown, known as hyperfibrinolysis, there can be a negative impact in the patient’s ability to survive. Tranexamic acid (TXA) is a synthetic derivative of the amino acid lysine that was created to serve as an antifibrinolytic agent.4 5 TXA acts by binding to plasminogen and blocking the interaction of plasminogen with fibrin, thereby stopping dissolution of the fibrin clot and preventing or reducing hemorrhage.2 4 6 TXA is an inexpensive yet effective treatment for patients with severe blood loss and it has been used in many settings, including during surgical procedures, dentistry, pregnancy, menstrual bleeding, hemophilia and trauma.7–12 Previous studies investigating the use of TXA have demonstrated improved survival and decreased hemorrhagic events with TXA use with few side effects.13 14 Consequently, TXA is included on the WHO’s List of Essential Medicines.
Prior to 2010, TXA was administered to military trauma patients at the discretion of the physician, but the effectiveness of TXA prompted the Joint Trauma System (JTS) to establish a clinical practice guideline (CPG), which outlined TXA’s role and suggested use in severely injured trauma patients who require damage control resuscitation.15 Since establishing the CPG, there has been an increased use of TXA to treat service members injured in the line of duty. The first Military Application of Tranexamic Acid in Trauma Emergency Resuscitation study (MATTERs I) was the first to collect data on the effectiveness of TXA in a military setting and found that the drug was associated with improved survival in military trauma patients. Despite these findings, there were also small increases in the number of patients who experienced a major venous thromboembolic event (VTE), which is a composite variable defined as having had a confirmed deep vein thrombosis (DVT) and/or pulmonary embolism (PE).14 Prior to the MATTERs I study, there was little documentation concerning the incidence of VTE among patients who were administered TXA.8 9 13 16–18 Following MATTERs I, recent investigations have determined that TXA is an independent risk factor for VTE in both military19 and civilian20 trauma patients. Furthermore, in an analysis of the military efforts from 2011 to 2015, Johnston et al determined that TXA is overused by military medical personnel. Given this information, military members represent a unique subset of the population that is at an increased risk of experiencing severe injury and VTE. We aim to establish the frequency with which overall VTE, DVT and PE were reported in severely injured US soldier trauma patients during Operation Enduring Freedom (OEF) (series of conflicts occurring as part of the Global War on Terrorism between 2001 and 2014) as well as identify the predictors of VTE in those treated with and without TXA from 2009 to 2014.
Methods
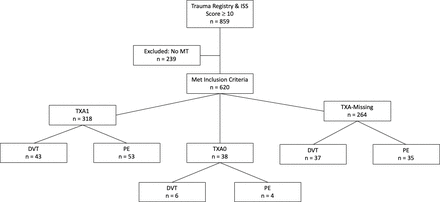
After obtaining Institutional Review Board approval, we conducted a retrospective, observational analysis using data from the Department of Defense Trauma Registry (DoDTR) data from 2009 to 2014. Within the sample population, measures for VTE prophylaxis were taken in accordance with the JTS CPG and included a compression device in addition to low-dose unfractionated heparin (5000 U) or low molecular weight heparin such as enoxaparin 30 mg, dalteparin 5000 U or enoxaparin 40 mg administered subcutaneously.15 In the event of traumatic brain injury, it was recommended that a neurosurgeon be consulted and a stable CT scan be assessed. All cases of VTE were identified and treated according to the JTS CPG, which required ultrasonography-confirmed or CT-confirmed DVT and/or PE.15 Those identified as having a VTE were treated with either anticoagulation or insertion of an inferior vena cava filter. Previous investigation has shown that the administration of TXA is most beneficial with severe injuries.14 In order to identify the most severely injured trauma patients, our inclusion criteria required that individuals had both an injury severity score (ISS) ≥10 and a massive transfusion (MT). ISS rankings were designated on a scale of 0–75 by taking into account total body injury, whereas MT was defined as having received ≥10 units of packed red blood cells (PRBC) and/or whole blood (WB) in the first 24 hours after injury.21 The DoDTR was queried to identify all US military injured in OEF from 1 January 2009 through 31 December 2014 with an ISS ≥10 resulting in a total of 859 subjects (figure 1). From this subset, 239 individuals did not meet the criteria for a MT and were excluded from the study. The remaining 620 eligible cases were separated into treatment groups based on reported TXA administration as determined by a TXA ‘yes’ or ‘no’ designation in the patients’ medical charts. Due to the nature of medical reporting, 264 cases had blank or missing TXA fields (TXA-Missing). There were 318 cases that reported ‘yes’ TXA (TXA1) and 38 confirmed cases of ‘no’ TXA (TXA0). The demographic information and group characteristics are listed in table 1. Our primary outcome measure was the occurrence of a VTE during the patient’s course of treatment.
Demographic data and group characteristics for all trauma patients with a MT and ISS >10 who received TXA, did not receive TXA and those who were missing TXA information from 2009 to 2014
Study profile of participant selection, study groups and primary outcome results. DVT, deep vein thrombosis; ISS, injury severity score; MT, massive transfusion; PE, pulmonary embolism; TXA1, tranexamic acid administered during treatment; TXA0, tranexamic acid not administered during treatment; TXA-Missing, tranexamic acid administration information is missing from medical chart.
Statistical analysis
In order to determine if the administration of TXA is an influential predictor of VTE, we used the logistic regression model:

where Y is 1 if a VTE occurred and 0 otherwise, and  represent the predictor variables:
represent the predictor variables:
TXA1;
Tourniquet;
First 24 hours transfusion: (whole blood, PRBC, platelets, cryoprecipitates and fresh frozen plasma);
Estimated blood loss first 24 hours;
Age;
Service branch (air force, army, marine and navy);
Injury year;
Mechanism of injury (bullet/gun shot wound/firearm, crush, explosive, helicopter crash, knife or sharp object, machinery/equipment, motor vehicle crash, pedestrian and plane crash);
Injury type (blunt, burn and penetrating);
Composite ISS;
AIS (head/neck, face, chest/thorax, abdomen, extremities and skin);
Days in intensive care unit;
Days on ventilator;
Days in hospital.
The least absolute shrinkage and selection operator (Lasso) method22 was used for estimating the model. In the Lasso method, all predictor variables are standardized so that each will have a mean of zero and a standard deviation (SD) of 1. The parameters 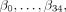 are constrained so that
are constrained so that
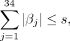
where s is chosen via cross-validation. With this approach, predictor variables with the largest influence on VTE will have coefficients with larger magnitude. Predictor variables that have little or no influence on VTE will have coefficients with magnitude close to or equal to zero. We can see the influence of the predictor variable on the OR of VTE by taking the natural exponentiation of the estimated coefficient.
All analyses were conducted in R V.3.5.3 using the glmnet,23 mice24 and VIM25 packages.
Results
Out of 620 severely injured trauma patients, 318 were confirmed TXA1, 38 were confirmed TXA0 and 264 were designated TXA-Missing. There were a total of 178 VTE in 169 individuals, including 86 DVT and 92 PE events reported in our sample. Eighteen individuals were identified as having developed both a PE and a DVT.
Dealing with missing data
Given the nature of a retrospective investigation across multiple centers, we thought it was important to perform a missing data analysis. As demonstrated in figure 2, the first 24 hours transfusion of frozen PRBC is missing in over 70% of the observations. The other two variables that contain missing values are TXA1 (42.6% missing) and the estimated blood loss first 24 hours (1.5% missing). Of the missing values for TXA, 98.5% were also missing the first 24 hours transfusion of frozen PRBC. Of the missing values for estimated blood loss first 24 hours, 33.1% were also missing first 24 hours transfusion of frozen PRBC. There were no observations where both TXA and estimated blood loss first 24 hours were missing. With such a large proportion missing, the first 24 hours transfusion of frozen PRBC was not included in the analysis.
To deal with the large amount of missing TXA information, the cases with missing values must either be removed or imputed via an imputation technique. The decision is based on the mechanism of missingness. In order to justify removing the cases with missing values, we must assume the mechanism is missing completely at random (MCAR). In MCAR, the reason for the missing values must be unrelated to any of the study variables. As with most applications, MCAR would not be appropriate for TXA in this dataset. If MCAR cannot be assumed, then the mechanism of missingness that is commonly assumed is missing at random (MAR). In MAR, the missing values are related to the study variables. Since the missing values are related to the other study variables, then imputation can be used by fitting a model that will explain the variable with missing values based on the other variables. One way to perform this imputation is with multiple imputation using chained equations (MICE).26 In table 2, we show the counts and percentages of the TXA1 and TXA0 variables for the complete cases along with the counts and percentages of TXA1 and TXA0 averaged over 10 imputed datasets using MICE. The differences in the percentages indicate that TXA is related to the other study variables and MCAR is not appropriate. We will analyze the data when MCAR assumed and when MAR assumed. It may be the case that the missingness in the TXA variable is due to the subject not receiving TXA (TXA0) thus nothing was recorded. In some cases, TXA was recorded as not being administered (TXA0) so we cannot be certain this is the reason for the missingness. Therefore, we will analyze the case in which the missing values are zeros as well.
Counts and percentages of TXA in the complete cases and for the imputed missing values
We first analysed the data using TXA1 and TXA0 with the TXA-Missing values removed. We then compared this with an analysis for which all of the TXA-Missing values were assumed to be TXA0 (TXA_noblank). Lastly, we used multiple imputation to predict TXA-Missing values.
Using TXA with missing data removed
The estimated parameters using the Lasso method are found in table 3 along with the corresponding effect on the OR of composite VTE to no VTE. The estimates are presented in descending order of their magnitude. We also estimated the model with PE and DVT separately as the response variable. The results of the Lasso fit show that TXA administration was not associated with incidence of PE, DVT or VTE.
Parameter estimates using the Lasso method and the corresponding effect on the OR when using TXA1
Using TXA_noblank
The estimated parameters using the Lasso method when TXA-Missing variables are treated as TXA0 are found in table 4. The estimates are presented in descending order of magnitude. We also estimated the model with PE and DVT separately as the response variable. As with the case of removing missing values, the Lasso fit results in zero coefficients for TXA for PE, DVT and VTE. These results indicate that TXA administration is not associated with incidence of PE, DVT or VTE.
Parameter estimates using the Lasso method and the corresponding effect on the OR when using TXA_noblank
Imputing missing values
Rather than assuming the missing values in TXA-Missing are missing because the subject was not administered TXA, we imputed values for the TXA-Missing cases. Of the 264 cases with TXA-Missing, 198 were imputed as TXA1 using MICE.
The estimated parameters using the Lasso method when TXA-Missing are imputed using MICE are found in table 5. We note that TXA has a non-zero coefficient for PE, DVT and VTE, unlike the results when the missing values are removed and when the missing values are assumed to be zero. For PE, the effect on the OR is 1.094, which implies that those with TXA1 are 9.4% more likely to experience PE when controlling for the other predictor variables. For any VTE, the effect on the OR is 1.030, which implies that those with TXA1 are 3% more likely to experience a VTE when controlling for the other predictor variables. For DVT, the effect of OR is 0.919, which implies that those with TXA1 are 8.1% less likely to experience DVT when controlling for the other predictor variables.
Parameter estimates using the Lasso method and the corresponding effect on the OR when imputing values with MICE for missing values of TXA1
Discussion
Using three separate logistic regression models, we analyzed the rate of VTE in severely injured individuals (n=620) who were treated during OEF. We examined a unique population of military trauma patients who had an ISS ≥10 and had received a MT. In contrast to previous studies, our study population had a significant amount of missing TXA information. Historically, statistical analyses of DoDTR data have used listwise deletion to deal with missing data, which can introduce excess bias. In order to avoid the pitfalls of listwise deletion and conduct a more thorough analysis of the dataset, we provided analyses which dealt with missingness in three distinct ways. We included a traditional approach to dealing with missing data through listwise deletion (TXA with missing data removed). Additionally, the TXA_noblank analysis dealt with missing data by systematically replacing missing values with TXA0, assuming missing information implied that no TXA was given. The analysis using multiple imputation dealt with missing data by using MICE. Using three separate logistic regression models, we determined the influence of TXA and other predictors on the outcome of VTE while controlling for injury mechanism, location and severity.
In all analyses, the mechanism of injury and injury type were more predictive of a VTE than TXA when holding all other variables constant. However, the logistic regression using multiple imputation demonstrated the greatest effect for TXA1, indicating that those who received TXA (TXA1) and those who were predicted to receive TXA (imputed as TXA1) had a 3% greater odds of experiencing a VTE and a 9.4% increased odds of PE, yet had an 8.1% reduced odds of DVT, after controlling for other variables. The MATTERs I study,14 Howard et al,27 Johnston et al 19 and Myers et al 20 suggest that TXA use is associated with an increased odds of VTE for those who also had a MT. Due to the mechanism of TXA’s influence on coagulation, it is plausible that higher rates of VTE could be produced by the drug. Multiple studies have noted that TXA is typically administered to individuals with more severe injuries who may not have survived without TXA.13 14 27 Previous results should be interpreted with caution because DVT and PE rates may be attributable to the higher ISS and overall injury burden in those treated with TXA. In our study, injury burden (measured by ISS) was similar across all levels of the study. The TXA0 and TXA1 groups had equivalent ISS (p=0.595) indicating that, on average, those who received TXA were no more severely injured than those who did not. TXA administration did significantly improve coagulopathy (p=0.049), as indicated by a smaller amount of estimated blood loss (884.5 mL less in TXA1 vs TXA0) which is congruent with the findings of the MATTERs I study.14
Although previous studies have assessed the efficacy of TXA, the status of ISS and MT are rarely specified. The Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage 2 study (CRASH-2), the largest TXA clinical trial to date, did not include injury severity data and only half of the subjects received a transfusion or required an operation.13 14 In military application, the ISS ranks the sum total of injuries on a scale from 0 to 75. The ISS ranking takes into account the notion that an accumulation of multiple non-fatal injuries can ultimately result in mortality, even when the individual injuries themselves may not be fatal.21 The JTS CPG15 indicates that individuals with more severe injuries, such as those who had a higher ISS and/or required a MT, should receive TXA. Previous studies in military settings have suggested controlling for injury severity and transfusion status in order to reduce confounding.14 27 Therefore, our inclusion criteria required that individuals had an ISS ≥10, which excluded those with less severe injuries. Additionally, in accordance with the MATTERs I study recommendation, we further excluded individuals who did not receive a MT (defined as having received ≥10 units of PRBC and/or whole blood) within the first 24 hours after injury. The inclusion criteria allowed us to assess VTE in the TXA1 and TXA0 groups while controlling for a high injury severity across all groups. Consequently, our study population had a sizeable proportion of VTE occur as compared with previous literature. We hypothesize that the increased rate of VTE was due to our strict inclusion criteria.
According to the CRASH-2 trial, the benefit of TXA is greatest when it is administered in the first 3 hours post-injury.13 Our study is limited by the observational nature of the DoDTR query results, which did not provide specific treatment information, such as the timing and use of VTE prophylaxis, TXA administration, other medications ordered for each patient or time of VTE. Therefore, it is likely that TXA could have been administered on different time scales for each subject, potentially biasing our results. Having specific timed data could have strengthened any association between TXA and VTE. Due to the nature of the observational DoDTR data, we do not have information regarding number and type of operative interventions. Additionally, we were limited by the large amount of missing data (blank fields), where TXA1 and TXA0 status was not designated. Since we are unable to discern if the TXA field was MCAR or MAR, we attempted to overcome this bias by using multiple strong statistical methods. We created three separate logistic regression models for each outcome variable, one of which used multiple imputation to deal with missingness in the TXA values. It is important to note that there is a limitation to using multiple imputation versus listwise deletion of the TXA-Missing values. With multiple imputation, the statistical analysis imputed a large number of TXA1 values (n=181), where TXA-Missing might have been TXA0. This led to different results than the analysis that used listwise deletion of the missing values. Finally, it is important to note that military members must fit within a defined age range and meet fitness standards to serve. While our sample is representative of the four branches of the military that were included in the analysis, our results may not be translatable to civilian trauma centers with more diverse populations.
We focused on a novel population of subjects who received a MT and had a high mean ISS relative to previous studies. Since the analysis of TXA1 versus TXA0 with no imputation requires no assumptions, we can conclude that it is the most unbiased result. In this analysis, we did not find that TXA was associated with PE, DVT or VTE. Nevertheless, given the nature of an antifibrinolytic drugs such as TXA, there is a possibility that it can precipitate a VTE. Further exploration of the risks and benefits of TXA is needed to determine if and when TXA use is warranted in severely injured military populations. Future studies would benefit from studying TXA in a prospective, randomized manner where timing of TXA and the subsequent outcomes (ie, DVT and PE) could be assessed along with any long-term consequence of TXA usage.
Conclusion
In our sample of severely injured military trauma patients, we demonstrated that TXA administration was inconsistently reported in military medical practice. Models utilizing multiple imputation to deal with missing data indicated that TXA use with an ISS >10 and MT resuscitation resulted in an increased odds of VTE and PE. The odds of DVT were found to be decreased after multiple imputation analysis. There is a need for continued education and training regarding TXA use and reporting. While causation cannot be determined from this cross-sectional analysis, further research regarding the risks and benefits of TXA in severely injured military populations is warranted.
Acknowledgments
The authors would like to thank James K Aden, Julie A Rizzo, Peter W Grandjean, Mitch Cholewinski and staff of the Department of Defense Trauma Registry (DoDTR).
References
Footnotes
Contributors KEA: literature search, statistical analysis, writing and revision. JDP: study design, statistical analysis, interpretation of data, writing. EJK: study design, data collection, writing. MNP: review, editing. SRH: study design, data collection, writing, review, editing.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement All data is available through the Department of Defense Trauma Registry.