Article Text
Abstract
Background The development of acute kidney injury (AKI) in trauma patients has been associated with an almost threefold increase in overall mortality. Many risk factors of mortality in severe AKI have been reported, but majority of the studies have been performed using a single-center data or have a small sample size. The purpose of this study was to identify the risk factors of mortality in severe AKI trauma patients.
Methods The study was performed using 2012-2016 American College of Surgeon Trauma Quality Improvement Program data, a national database of trauma patients in the USA.
All adult trauma patients aged 16 to 89 years old, admitted to the hospital and who developed a severe AKI were included in the study. A p value of <0.05 was considered statistically significant.
Results Out of 9309 trauma patients who developed severe AKI, 2641 (28.08%) died. There were significant differences found in bivariate analysis between the groups who died and who survived after developing a severe AKI. Multivariable analysis showed male sex, older age, higher Injury Severity Score, lower Glasgow Coma Scale, presence of hypotension (systolic blood pressure<90 mm Hg) and coagulopathy were all significantly associated with in-hospital mortality. The area under the curve value was 0.706 and the 95% CI was 0.68 to 0.727.
Discussion Current analysis showed certain patients’ characteristics are associated with higher mortality in patients with severe AKI. Prompt identification and aggressive monitoring and management in high-risk patients may result in reduced mortality.
Level of evidence IV.
Study type Observational cohort study.
- multiple trauma
- acute kidney injury
- mortality
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
The mortality rate among patients with traumatic injuries varies, depending on the cause of injury, with reported rates ranging from as low as 0.4% to ~27% for traffic-related injuries or suicide attempts.1 2 Concomitant medical disease is an important contributor to mortality risk among trauma patients. For instance, Koh et al2 examined the cause-specific mortality among trauma patients in a single US institution across two separate time frames. Of all the causes identified, in the time frame 2012–2013, multiorgan failure/sepsis, cardiac and respiratory failure were three important causes of death (20.0%, 9.2% and 6.2% of all causes of death in 2012–2013, respectively). Another important morbid condition that develops among patients with trauma, especially the more severely injured, is acute kidney injury (AKI). Bihorac et al3 identified that nearly 25% of patients with severe traumatic injury developed AKI within 28 days of admission, but only ~9% developed severe AKI. Evaluations in larger datasets demonstrate a fairly low rate of severe AKI (0.99% among a national survey of all trauma centers in the USA).4 Despite a generally low incidence of severe AKI, the mortality rate in trauma patients is proportional to the severity of AKI (HR for in-hospital mortality relative to no AKI, of ~2.5 for mild to moderate AKI, and 4.5 for severe AKI; p=0.001 for trend).3 Another small study of US military personnel trauma victims demonstrated a mortality rate of 18% when severe AKI (that requiring renal replacement therapy, eg, hemodialysis) complicated the trauma.5 More recent analysis of the effect of severe AKI on the outcomes of trauma patients in larger datasets, specifically in the era of improved capability of providing renal support, has not been conducted. The purpose of this study was to evaluate the in-hospital mortality of those trauma patients who developed severe AKI, after a trauma using the Trauma Quality Improvement Program (TQIP) dataset.
Methods
The information of all injured patients with Injury Severity Score (ISS) scores of ≥4, who were brought to the hospital after a traumatic mechanism of injury and developed severe acute kidney injury (AKI) during the index hospital admission, were accessed from the TQIP database for the year 2012 to 2016. The TQIP is a quality improvement program. Trauma centers across the USA participated in this program. Currently, 825 institutions submit their patient’s information and the TQIP provides feedback to the participating centers.6 Severe AKI was defined as an abrupt decrease in kidney function either three times increase in serum creatinine (SCr) level from the baseline or increase in SCr to ≥4.0 mg/dL (≥353.6 µmol/L), initiation of renal replacement therapy or anuria for ≥12 hours .7 Included in the study were all adult patients’ ages 16 to 89 years old who sustained injury and were hospitalized. Other variables included patients’ demography, initial vital signs (systolic blood pressure (SBP) of <90 mm Hg) (hypotension), heart rate, injury type, injury characteristics; Injury Severity Score (ISS), Glasgow Coma Scale (GCS) score and comorbidities; hypertension (HTN), congestive heart failure and history of smoking, diabetes mellitus, respiratory disease, obesity, myocardial infarction and coagulopathy, including use of anticoagulation. Patients with history of chronic kidney disease, identified by diagnostic codes (ICD9CM 585 .x), were excluded from the study.
The primary outcome of this study was to identify the incidence of mortality and develop a risk model to predict the risk factors associated with in-hospital mortality in patients with severe AKI.
Statistics
The patient characteristics and outcomes were first summarized as median with IQR (first quartile to third quartile) for continuous variables, and frequency and percentage for categorical variables. The patients who died after developing a severe AKI were compared with the patients who survived. The two groups were compared using Wilcoxon rank-sum test for continuous variables, and χ2 test for categorical variables.
The variables used for performing the risk assessment were only those relevant variables who may have contributed directly to mortality8 and those variables that were highly significant in bivariate analysis that included age, race (white vs. nonwhite), sex (male vs. female), ISS, SBP of <90 mm Hg (hypotension) at initial presentation and history of coagulopathy, including use of anticoagulation. The primary purpose of building this model was to identify any significant associations between these critical clinical factors and in-hospital mortality. The modeling variable selection process combined prior literature considerations, clinical input, and both an initial stepwise-AIC process and a backward likelihood ratio process as confirmation of factor selection. For validating the risk assessment model, the data were divided into a training dataset, which contained 80% of the randomly selected data, and a testing dataset, which contained the other 20% of the data. A multiple logistic regression model was used to assess the chance of death with selected variables. The receiver operating characteristic (ROC) curve was constructed, and the corresponding area under the curve (AUC) was calculated. The parameter estimates from the fitted model were summarized using β coefficient estimates and 95% CIs for the odds ratio (OR) as measures of precision.4 The β coefficient for each variables was multiplied by a factor of 10 and rounded to the nearest whole number to achieve a score. The risk score was further tested for the predictability of the development of risk of mortality in severe AKI.
The two-sided p value was reported for each test. A p value of <0.05 was considered an indication of statistical significance. Statistical analysis was performed using the R language.9
Results
Baseline patient’s characteristics
Only patients who developed severe AKI were included in this analysis. A total of 9309 patients were identified. The median age of the cohort was 61 years old; more than 70% of the patients were Caucasians; and 73.6% were male. Majority of patients (89.2%) sustained blunt trauma. Fall and motor vehicle crashes were the two main mechanisms of injury found 38.6% and 27.3%, respectively. Most of the patients (83.3%) were admitted to the intensive care units. Diabetes and HTN were the two most common comorbidities found in this patient cohort.
Unadjusted bivariate analysis
Out of 9309 trauma patients who developed severe AKI, 2641 (28.08%) died and 6695 (71.92%) survived to discharge after initial hospital admission. In the bivariate analyses, there were significant differences found between the groups who died and who survived after developing a severe AKI. These differences were more pronounced in median age (62 years (IQR 46 to 76 years) versus 60 years (IQR 42 to 75 years)), male sex (77.4% vs. 72.1%), ISS (25 (IQR 16 to 34) vs. 17 (IQR 9 to 26)), GCS score (14 (IQR 3 to 15) vs. 15 (IQR 13 to 15)) and presence of hypotension at initial presentation (16.8% vs. 11.1%); all p values were <0.001 (table 1).
Bivariate analysis of patients with severe AKI who died and who survived
The only pre-existing condition that was found to be higher among patients who died was the history of coagulopathy, including prior use of anticoagulation (15.4% vs. 11.9% among those who died and survived, p<0.001). The presence of HTN, diabetes and prior smoking history were more frequent in those who survived as compared with those who died (p<0.05 for all). All other identified comorbidities were similar between the groups.
Multivariable analysis and risk modelling
A multivariable logistic regression model was then fit using those patient’s characteristics that were related to hospital mortality and or those characteristics of patients that were found to demonstrate a significant difference between the groups who died and who survived. Male sex, older age, higher ISS, lower GCS, presence of hypotension at initial presentation and history of anticoagulation use or bleeding disorder were all significantly associated with in-hospital mortality (table 2).
Risk factors of mortality in patients with severe AKI
Log odds of mortality=−2.72 + 0.17×male+0.12×not white+0.16×hypotension +0.40×bleeding disorder+0.41×age (if 45 to 54 years)+0.82×age (if 55 to 64 years)+0.97×age (if 65 to 74 years)+1.15×age (if 75 to 89 years)+0.58×ISS (if 15 to 24)+1.04×ISS (if 25 to 34)+1.35×ISS (if 35–44)+1.68×ISS (if 45 to 75)+0.83×GCS (if 3 to 8)+0.26×GCS (if 9–12).
Probability of mortality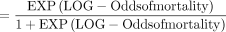
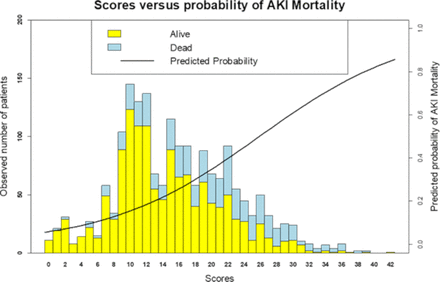
Our risk of mortality score ranges from 0 to 45. The mortality steadily increases with the score: the higher the score, the higher the mortality (please see table 3 and figure 1).
Risk of mortality score with severe AKI
Risk score versus predictability of mortality. AKI, acute kidney injury.
Model performance
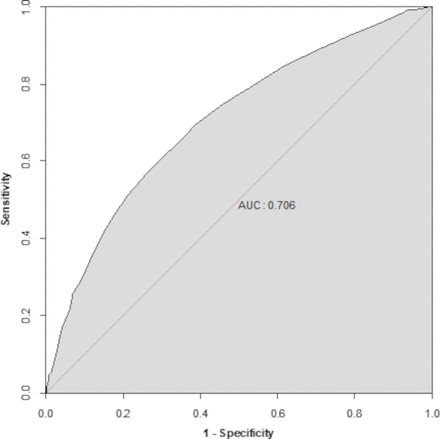
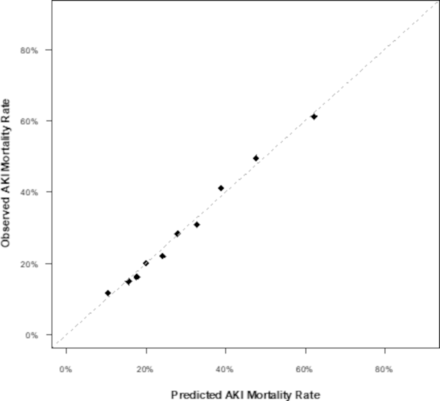
To assess the overall strength of the model for mortality, an ROC curve was then fit using the testing dataset (figure 2). The model showed a fair strength with an AUC value of 0.706, and the 95% CI was 0.68 to 0.727. An evaluation of the calibration of the model was also performed to assess the validity of the model by comparing the observed events versus the expected event rates (figure 3). The figure demonstrates an adequate comparison between observed and predicted rates of in-hospital mortality.
ROC curve for prognostic model performance for predicting mortality in severe AKI. AKI, acute kidney injury; AUC, area under the curve; ROC, receiver operating characteristic.
Observed versus fitted average mortality in severe AKI. AKI, acue kidney injury.
Discussion
In one of the largest cohorts in which contributors of mortality associated with severe AKI in trauma patients could be examined, we have identified a high mortality among patients with severe AKI in the setting of trauma, as well as important risk factors for mortality.
AKI is a common event among trauma patients, though the true incidence may vary, depending on the method used to identify AKI and the stage of AKI considered, with more severe forms of AKI being less common.3 4 10–13 Nevertheless, the occurrence of AKI is associated with a high rate of mortality with estimates ranging from as low as 7% (for lowest severity of AKI) to as high as 69% in the highest severity stage of AKI (often requiring dialysis support).13 Our analysis is consistent with these prior reports. In our cohort, patients with AKI were identified if they met the highest stage of AKI or required renal replacement therapy. In this severe AKI group, mortality was found to be 28%.
Identifying risk factors, especially if potentially modifiable, will be important in implementing risk reduction measures for outcome improvement in this high-morbidity subset of trauma patients. In our cohort, factors identified that correlate with mortality in severe AKI and trauma include older age, higher severity of trauma, male gender, lower blood pressure, and bleeding diathesis or history of anticoagulation use. Most of these factors are non-modifiable. The association of older age is a relative association. The majority of patients who die with AKI in the setting of trauma are still fairly young: the median age of those who died in our cohort was 62 years (range 46 to 76 years). Perkins et al12 examined the mortality rate associated with any AKI stage in a trauma center located in Brazil. Mortality was 26.4% in those with AKI and 10.4% in those without AKI. The mean age in those with AKI was 44 years versus 35 years in those without AKI. Similarly, Bolanos et al5 reported on outcomes of severe AKI requiring renal replacement therapy in young (mean age 26 years) military personnel after battlefront injury and found 22% 60-day mortality. Thus, the effects of AKI on mortality risk in trauma patients is still relevant in younger patients.
The presence of hemodynamic instability is a potentially modifiable risk factor. The presence of SBP of less than 90 was associated with an increased risk of mortality in our cohort. Hemodynamic instability can be targeted quickly and effectively with volume and pressor support, but in the trauma patient may represent significant internal injury and/or bleeding that may independently put patients at risk of mortality.11 There are no studies currently that have looked at specific hemodynamic targets or mechanisms of hemodynamic support in improving outcomes in trauma patients with severe AKI. On the other hand, there are some emerging data regarding hypotensive resuscitation in hemorrhagic shock. A recent meta-analysis of the current evidence on this subject suggests that hypotensive resuscitation in the appropriately selected patients may have some mortality benefit without excessive risk of AKI.14 Another approach to hemorrhagic trauma patients can be damage control resuscitation, which includes controlling of hemorrhage, replacement of lost blood and preventing coagulopathy can be applied to reduce the incidence of mortality.15 Thus, more evaluation of the interaction of hemodynamics and AKI in trauma patients is required to identify appropriate management measures around these characteristics.
Important limitations of the present analysis need to be acknowledged. The requirement for dialysis support is known to increase the risk for adverse outcomes in AKI, including that in trauma.13 The breakdown of patients who did and did not require Renal Replacement Therapy (RRT) for AKI was not available in this analysis. Including all patients in the American College of Surgeon TQIP dataset may have included patients with essentially such severe trauma that death would not have been preventable, and these patients would be more likely to have AKI, given the association with severity of trauma as seen in the current and prior analysis.2 4 Finally, novel biomarkers are available that can help with better detection of AKI in trauma patients and may help with risk prediction.16 Such information was not available for this analysis. The exact cause of death in patients with severe AKI was not available from the database.
Despite these limitations, we have validated findings from smaller studies in a large representative dataset of trauma victims within the USA. This information can be used to identify higher-risk individuals who may benefit from potential preventive strategies or for inclusion into studies testing such strategies. Some of the strategies may include aggressive resuscitation and monitoring of high-risk patients in a critical care setting. For example, management of patients with severe AKI in critical care setting and intensive monitoring of urine output yielded a better survival outcome.17
Conclusion
In a large, nationally representative cohort of patients with trauma and severe AKI, we have demonstrated a current representative mortality risk estimate. In addition, we have identified several significant risk factors that are associated with mortality among patients with severe AKI in trauma. Further research is warranted to identify means to improve mortality outcomes in this high-risk population.
Footnotes
Contributors NA conceived and designed the study and was responsible for retrieving the study data, and YHK performed the data analysis. NA, YHK, RM and AA interpreted the data and significantly contributed to the writing of the article.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not applicable.
Ethics approval All procedures followed were accordance with the ethical standards of the institutional review board (IRB) of Meridian Health and with the Helsinki Declaration of 1975, as revised in 2008. Since the data of Trauma Quality iImprovement Program are deidentified patient’s information available to the researchers, the study was exempted from Meridian Health IRB review.
Provenance and peer review Not commissioned; internally peer reviewed.
Data availability statement Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as supplementary information. Deidentified participant data from the Trauma Quality Improvement Program database are available from the American College of Surgeons.