Article Text
Abstract
Anticoagulant-associated traumatic intracranial hemorrhage (tICrH) is a devastating injury with high morbidity and mortality. For survivors, treating clinicians face the dilemma of restarting oral anticoagulation with scarce evidence to guide them. Thromboembolic risk is high from the bleeding event, patients’ high baseline risks, that is, the pre-existing indication for anticoagulation, and the risk of immobility after the bleeding episode. This must be balanced with potentially devastating hematoma expansion or new hemorrhagic lesions. Retrospective evidence and expert opinion support restarting oral anticoagulants in most patients with tICrH, but timing is uncertain. Researchers have failed to make clear distinctions between tICrH and spontaneous intracranial hemorrhage (sICrH), which have differing natural histories. While both appear to benefit from restarting, sICrH has a higher rebleeding risk and similar or lower thrombotic risk. Clinical equipoise on restarting is also divergent. In sICrH, equipoise is centered on whether to restart. In tICrH, it is centered on when. Several prospective randomized clinical trials are ongoing or about to start to examine the risk–benefit of restarting. Most of them are restricted to patients with sICrH, with antiplatelet control groups. Most are also restricted to direct oral anticoagulants (DOACs), as they are associated with a lower overall risk of ICrH. There is some overlap with tICrH via subdural hematoma, and one trial is specific to restart timing with DOACs in only traumatic cases. This is a narrative review of the current evidence for restarting anticoagulation and restart timing after tICrH along with a summary of the ongoing and planned clinical trials.
- brain injuries
- traumatic
- anticoagulants
- thromboembolism
- hemorrhage
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Background
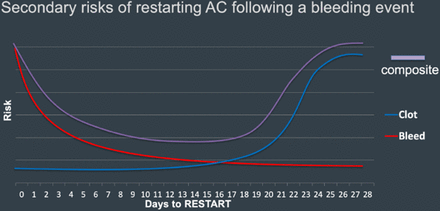
US trauma centers care for >18 000 anticoagulated traumatic intracranial hemorrhages (tICrHs) per year,1 although this likely understates the disease scope as many tICrH cases are cared for in non-trauma centers.2 Failure to restart anticoagulation in tICrH survivors contributes to an enormous thromboembolic burden in strokes, venous thromboembolism (VTE) and other events.3–5 The risk–benefit in the naïve state usually favors anticoagulation by an order of magnitude, but this relationship shifts once ICrH has occurred,3 5 when clinicians must cease or reverse anticoagulation to stabilize the patient. At some time point, most patients with tICrH return to a state which again favors anticoagulation. A survey of neurosurgeons found nearly half encounter the tICrH restart question once a week or more.4 There is already broad consensus that most patients with tICrH should be restarted.3 4 The unanswered question is when.3 4 In the absence of randomized trials and a definitive time target, restart practice in tICrH is highly variable—from 3 days to several months to never.5 Cumulative thromboembolic risk rises with time and is a combination of baseline risk, the bleeding episode itself, any reversal agents used and immobility afterwards. Rebleeding risk is high early when clots are forming and stabilizing and falls with increasing time (see figure 1), eventually to a baseline, which may be informed/adjusted by the type of index bleeding episode for any additional rebleeding risk. Importantly, ICrH is at least two diseases with different natural histories. Spontaneous intracerebral hemorrhage (sICH), such as lobar ICH with cerebral amyloid angiopathy, may heighten the patient’s risk for recurrent bleeding and alter the baseline risk–benefit for anticoagulation.6 In contradistinction, after tICrH, the index hemorrhage is less likely to affect the patient’s baseline bleeding risk.7 Several trials are in planning or actively enrolling to address anticoagulation restart and restart timing questions. Numerous retrospective cohorts have found benefit with minimal increased risk of recurrent hemorrhage in patients with restarted tICrH.5 7–11 This narrative review summarizes the retrospective evidence for restart and timing of restart of oral anticoagulants after tICrH, the guidelines and the prospective trials on this topic.
Conceptual representation of stratified results of secondary risks of restarting anticoagulation following a bleeding event, over time to restart. AC: anticoagulation.
Methods
The evidence for this review was obtained through searches of PubMed between January 1, 2010 and January 1, 2020, supplemented with a review using Google Scholar (Alphabet). The systematic search excluded evidence from prior to 2010 because this evidence would focus almost exclusively on treatment and reversal with vitamin K antagonist (VKA) treatment. The searches were conducted using the structured search terms: traumatic AND (intracranial OR intracerebral) AND hemorrhage OR “traumatic brain injury” AND anticoagulation within all available searchable fields.
Titles and abstracts were reviewed for inclusion in the review based on relevance to the central research questions. Included articles addressed anticoagulation-associated ICrH presentations and treated the question of treatment reinitiation within the results reported in the abstract. The review excluded search results that did not address the principle questions in this review, addressed conditions other than ICrH without stratified analysis of this condition, did not consider anticoagulation or exclusively considered antiplatelet treatments or did not evaluate treatment reinitiation (either decision or timing). The review excluded case studies, series, and qualitative research studies as well.
In addition, PubMed and Google Scholar were searched for clinical guidelines relevant to the clinical topic. This search was conducted using the structured search terms: “guidelines”[Title] AND (“intracranial hemorrhage” OR “intracerebral hemorrhage” OR “traumatic brain injury”). Guidelines obtained were reviewed to confirm that they represented national or international experts and that the committees were represented by appropriate professional organizations. Trials were identified at international meetings and through personal contacts and confirmed as registered on ClinicalTrials.gov.
Results
The review of PubMed returned 509 articles, with 17 retained following title and abstract review. Searching available clinical guidelines provided 3 relevant guidelines (out of 184 results) and 12 additional references. The remaining references (24) were identified from review of pertinent bibliographies and Google Scholar.
Equipoise
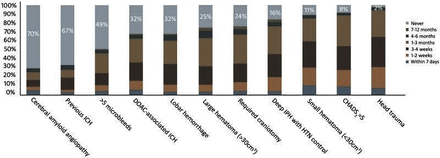
Much of the available evidence on restart of anticoagulation therapy after ICrH comes from sICrH data. In this context, the equipoise is on whether (rather than when) to restart anticoagulation. In tICrH, primarily cared for by trauma surgeons and neurosurgeons, the equipoise is shifted to when to resume anticoagulation in most patients. Clinicians who care for tICrH see the traumatic episode as largely isolated, perhaps influenced by fall risk, although only to a very small degree (295 falls per year to offset anticoagulant benefit).12 Otherwise, it is largely disconnected from the baseline risk–benefit ratio for anticoagulation. A global survey of 228 neurologists, neurosurgeons and thrombosis experts found 98% believed patients with tICrH should be restarted (see figure 2) but demonstrated wide variability as to when, noting ‘randomized trials are direly needed in this population’.4 In another neurosurgical survey, approximately 80% chose restart timing intervals of 1 week or 1 month (vs never or longer) in central nervous system hemorrhage.3 The differing equipoise in sICrH and tICrH is illustrated in the prospective trial designs (see ‘Prospective randomized trials’ section for details). The one tICrH trial is focused on timing of restart without a non-anticoagulated control, while the several sICrH trials all have a control group.
Overall response from survey participants on timing of oral anticoagulant re-initiation across 11 clinical scenarios.4 Reproduced with open access from Xu et al.4 2018 Public Library of Science under CC BY 4.0. DOAC, direct oral anticoagulant; HTN, hypertension; ICH, intracerebral hemorrhage; IPH, intraparenchymal hemorrhage.
Clinical guidelines
Current trauma guidelines do not address the use of anticoagulant treatment after ICrH. The 2014 update to the American Heart Association/American Stroke Association guidelines for stroke prevention in stroke and temporary ischemic attack includes a special section on the use of antithrombotic therapy after ICrH.13 These guidelines clearly demonstrate shortcomings of the available evidence as they endorse individualized assessment of the restart decision. To this end, they advise consideration of the risks of subsequent thromboembolism, recurrent ICrH and overall ‘patient status’. It subsequently suggests that cases with higher risk of recurrent ICH relative to cerebral infarction may consider antiplatelet therapy instead of anticoagulation. With regard to timing of anticoagulation reinitiation, ‘the optimal timing is uncertain. For most patients, however, it might be reasonable to wait ≥1 week’.13 None of the evidence provided rises above class IIb, level B in strength.
The European Stroke Organization also released guidelines in 2014 for sICH, which recognize a lack of trial evidence on this subject.14 The guidelines state that a firm recommendation about whether or when to resume antithrombotic medication after ICH cannot be made, and rate the evidence as ‘very low’ quality. The timing suggestions reviewed by that committee range from 14 days up to 30 weeks.14 15
An Austrian expert-consensus panel focused exclusively on traumatic brain injury found that there was ‘insufficient evidence to support or discourage the resumption’ of oral anticoagulation following tICrH and that clinical decisions should be made on a ‘case-by-case basis’.16 In spite of this, the practice of restarting anticoagulation in tICrH is far more common than with spontaneous cases as the decision of whether to resume treatment appears already to have been answered in most patients with tICrH and transformed into clinical practice.3 4
Evidence: natural history of anticoagulated tICrH and hematoma expansion
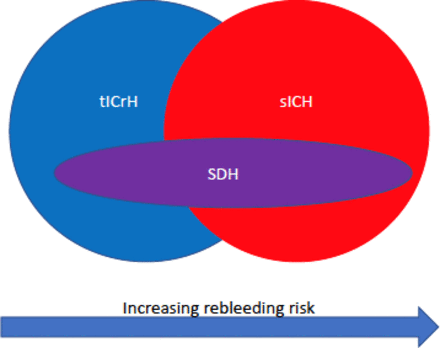
The literature on anticoagulated tICrH is cross-contaminated with sICrH data. Investigators have not rigorously applied specific nomenclature. The abbreviation ICH has been applied to intracranial hemorrhage, intracerebral hemorrhage, traumatic and spontaneous, all of the above and various subsets. (We use the preferred specific nomenclature: intracerebral hemorrhage is ICH, intracranial hemorrhage is ICrH. These are preceded by t or s for traumatic or spontaneous.) While there is considerable variation within sICrH and tICrH, it is becoming clear there is also a distinct natural history between them. There is less data on tICrH, but what exists seems to indicate a lower risk of recurrent hemorrhage than sICrH and a higher rate of thromboembolism.5 7–9 The possible exception is subdural hematoma (SDH) (see figure 3), which may carry a higher rebleeding risk than other tICrH.17 The natural history of tICrH as a disease is important in this context for understanding when the acute event ends, that is, the cessation of hematoma expansion and stabilization of clot. Anticoagulants do not break fibrin bonds. They prohibit the deposition of new fibrin by inhibiting various factors in the clotting cascade. Hematoma expansion in tICrH is very common in the first 24 hours, much less so by 48 hours and rare by 72 hours.18 19 SDHs occasionally expand later, several days to weeks, or develop into chronic SDH/hygroma, although late surgical intervention is required with diminishing frequency the longer it remains stable.20 It is clear from the literature that preinjury anticoagulation is associated with worse outcome, double the risk of death, increased risk of hematoma expansion (early and delayed) generally meaning after 24 hours.19 However, there was evidence from a small (n=63), prospective observational study of traumatic intracerebral hemorrhages that hematoma expansion slows from 24 to 72 hours, and may not extend beyond 3–4 days.20
Bleeding risk by intracranial hemorrhage subtype. SDH. subdural hematoma; sICrH, spontaneous intracranial hemorrhage; tICrH, traumatic intracranial hemorrhage.
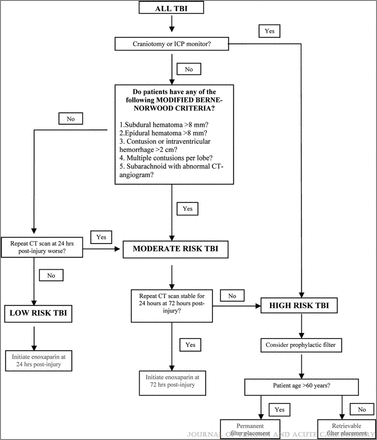
Trauma investigators have developed a decision rule to risk stratify patients with tICrH for early chemoprophylaxis (see figure 4).21 While this rule has only been tested in a pilot trial of low-risk patients with stable CT images for early VTE prevention (prophylactic dose heparins, not full oral anticoagulation), it is useful to conceptualize risk of hematoma expansion in the setting of anticoagulant considerations.21 It is also telling that the study of 62 patients had no clinically significant hematoma expansion at 48 hours after injury. Ongoing changes in hemorrhage based on follow-up imaging may offer an important criterion for evaluating patients for treatment reinitiation.
The Parkland Protocol (modified Berne Norwood criteria) categorizes traumatic brain injury (TBI) patterns as low, moderate or high risk for hematoma expansion when considering venous thromboembolism (VTE) prophylaxis. Reproduced with conditional permission from Phelan et al.21 Copyright 2012 Wolters Kluwer Health.
Risk of recurrent ICrH
With the exception of the relatively rare cases of traumatic pseudoaneurysm,22 the primary recurrent bleeding risk in tICrH beyond the acute period is the same as the primary cause of the index event: falls. Fall risk is a common reason clinicians withhold anticoagulation,23–25 but there is little evidence that it should preclude treatment.12 25 While approximately 5% of fall cases were readmitted for the same reason within 6 months, anticoagulation treatment was not associated with likelihood of a second fall or hemorrhage related to the fall in a nationally representative readmission registry26 as well as a Medicare database.27 However, anticoagulation was associated with disproportionate mortality in those who bled from secondary fall events (21.5% vs 6.9%).26 Secondary analysis of the ARISTOTLE trial (n=16 491) of apixaban and warfarin revealed that prior history of falls resulted in elevated risk of major hemorrhage, including ICrH, and death (adjusted HR (aHR) (95% CI)=1.39 (1.05 to 1.84), 1.87 (1.02 to 3.43), 1.70 (1.36 to 2.14), respectively) in anticoagulated subjects.28 Fall risk assessed during the ENGAGE-AF-TIMI48 trial of edoxaban and warfarin was associated with major bleeding, life-threatening bleeding and all-cause mortality (aHR (95% CI)=1.30 (1.04 to 1.64), 1.67 (1.11 to 2.50), 1.45 (1.23 to 1.70), respectively).29 In another study, however, elevated fall risk was not found to be associated with likelihood of major hemorrhage in a prospective cohort study of 515 patients taking oral anticoagulation.30 The risk of major hemorrhage after initiation of oral anticoagulation has been estimated numerous ways, but Donzé et al found that all major hemorrhages related to falls comprised just 0.6 of the 7.5 major hemorrhages per 100 person-years of follow-up.30 A study of 1245 Medicare beneficiaries with atrial fibrillation (AF) and at high risk for falls found a rate of 2.8 ICrHs per 100 person-years, 2.0 of which were traumatic in nature.27 This was significantly elevated above the rates for patients with AF not at risk of falls, of 1.1 and 0.34 per 100 person-years, respectively.27
Even considering the potential for elevated risk of major hemorrhage, ICrH and associated mortality, studies suggest that the benefits of treatment with anticoagulation outweigh any harms.25 A theoretical, clinical decision-analytic model was used to parse the optimal treatment strategy in a population of elderly patients with AF at risk for falling. Controlling for age and baseline stroke risk, implementing warfarin therapy (the only treatment available at that time) was associated with >2.5 greater quality-adjusted life-years than withholding antithrombotic treatment in elderly patients. Their analysis showed that a patient would need to fall approximately 295 times in a year to exceed the benefit of stroke prevention from anticoagulation therapy.12 In the optimization model, fall risk was not a relevant factor in the clinical decision to start treatment.12
There are scales to quantify the bleeding risk in the anticoagulated patients, such as HAS-BLED31 and HEMORR2HAGES32 and others. Creators note that the scales were primarily created to identify modifiable risk factors, for example, excessive alcohol use, not to exclude patients from anticoagulation treatment.31 32
Thrombotic risk in tICrH
The rebleeding risk in tICrH does not exist in isolation. It must be balanced with the thrombotic risk. There is much in the trauma literature on VTE risk in patients with tICrH, less on ischemic stroke and myocardial infarction (MI) and less still specifically on anticoagulated patients. The long-term risk can be estimated with the CHA2DS2-VASc score.33 However, the richest source of detail on short-term thromboembolic events in anticoagulated patients are the anticoagulant reversal trials, as those events were primary safety outcomes.34–36 The four-factor prothrombin complex concentrate (4F-PCC) for warfarin reversal trial was only 10% ICrH.34 The idarucizumab trial for dabigatran reversal was 25% ICrH.34 The andexanet alfa trial for factor Xa inhibitor reversal was 64% ICrH (242 of 352, 99 of which were tICrH).35 These trials are important for several reasons. All the patients were anticoagulated. Most (80%) were on the drug for AF. All were followed carefully for thrombotic events, including stroke, MI and VTE, for 30 days with site monitoring, independent academic adjudication of events and federal Food and Drug Administration oversight. The 30-day thrombotic event rates in reversal trials were 5% (idarucizumab), 6% (4F-PCC) and 10% (andexanet).34 35 37 Except for the earlier 4F-PCC trial, there was a significant amount of restarting of full oral anticoagulation, for example, 28% in andexanet, none of whom suffered a thrombotic event thereafter. In contrast, literature specific to tICrH (but not to preinjury anticoagulant status) puts the short-term thrombotic event rate much higher, >20%.36
Restart studies specific to tICrH
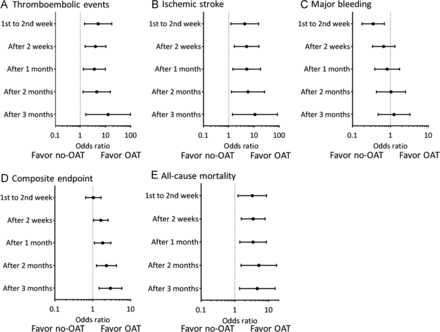
There are analyses and meta-analyses using billing data and prescription databases to establish the risk–benefit of restarting anticoagulation after ICrH in general and tICrH specifically.5 7 38 A recent large retrospective analysis underscores the potential benefit. Nielsen et al gathered a sample of 1325 patients with sICrH and 1090 patients with tICrH.7 Resuming anticoagulation in patients with sICrH was associated with a 51% decrease in stroke or systemic embolism but a 31% increase in recurrent ICrH (neither statistically significant). Resuming anticoagulation in patients with tICrH was associated with a 60% reduction in stroke or systemic embolism (not statistically significant) and a 55% reduction in recurrent ICrH, which was statistically significant (aHR 0.45; 95% CI 0.26 to 0.76). Both groups saw significant reductions in all-cause mortality, 49% and 55% in sICrH and tICrH, respectively (sICrH aHR 0.51; 95% CI 0.37 to 0.71) and (tICrH aHR 0.35; 95% CI 0.23 to 0.52).7 A reduction in recurrent ICrH with anticoagulation may seem non-sensical. It probably represents selection bias of restarting lower bleeding risk patients, but it certainly is more reassuring than a null or increased bleeding risk finding in this biased setting. Albrecht et al used Medicare claims data to identify anticoagulated patients with traumatic brain injury and restarting outcomes.5 They found a decreased risk of thrombotic events (relative risk (RR) 0.77 (95% CI 0.67 to 0.88)), an increased risk of hemorrhagic events (RR 1.51 (95% CI 1.29 to 1.78)) and a decreased risk of combined hemorrhagic or ischemic stroke (RR 0.83 (95% CI 0.72 to 0.96)). Prescription data were unable to speak to timing with any precision. Park et al retrospectively analyzed 428 patients with AF with a history of ICrH, including SDH and epidural hemorrhage (EDH), although they did not distinguish whether intraparenchymal and subarachnoid hemorrhages were spontaneous, traumatic or mixed.8 The analysis did allow some inference on timing for a bleeding and thrombosis composite outcome (see figure 5), although with the usual caveats about retrospective design and selection bias and that it is a combination of patients with sICH and tICrH. Another retrospective study of antithrombotic treatment restart in 85 patients with traumatic brain injury showed that the lowest rate of secondary clinical events occurred in those started between 7 and 14 days, with the highest rate of events in those cases that never resumed treatment.39
HRs according to the timing of warfarin initiation. (A) Thromboembolic events; (B) ischemic stroke; (C) major bleeding; (D) composite end point; (E) all-cause mortality. OAT: oral anticoagulation therapy. Reproduced with permission from Park et al.8 Copyright 2016 Elsevier.
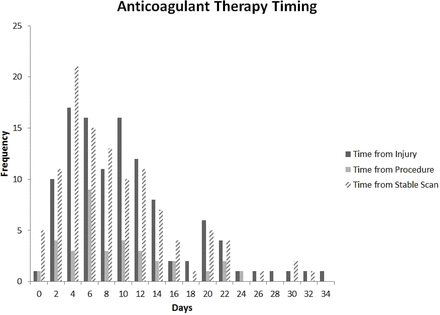
Three other studies focus on the immediate postacute period in patients with tICrH. Divito et al retrospectively analyzed 112 patients with tICrH, skewing severe with 29% requiring neurosurgical intervention. Median restart interval was 8 days (range 1–31) (see figure 6).10 These patients had 88 thromboembolic events: 32 pulmonary emboli (PEs), 44 deep venous thromboses (DVTs), 4 MIs and 2 left ventricular thrombi. To a large extent, the events in Divito drove restart decisions as opposed to starting with a priori secondary prevention in mind, and the population skewed severe with 42% having a Glasgow Coma Score <9.10 However, the bleeding risk seen is informative. There were no deaths directly attributable to anticoagulation. One patient developed a chronic subdural hygroma that required surgery 2 weeks later, and one patient died of a hemothorax, but the patient was also in septic shock and the family declined further intervention.10 Byrnes et al used a similar retrospective model in which the thromboembolic event drove anticoagulation, analyzing 42 patients with tICrH who developed DVT, PE or blunt cerebrovascular injury, 26 of which were anticoagulated.11 Mean start interval was 11.9 days with a broad range with nearly half restarted between 1 and 2 weeks. Byrnes et al did not report on thromboembolic events after initiation of anticoagulation and found only one case of hematoma expansion, 1–2 mm in an intraparenchymal hemorrhage 6 days after restart that did not affect clinical course. Pandya et al studied 97 patients with tICrH, dividing them into SDH and all other tICrH and correlating start of either anticoagulants 36%, antiplatelets (53%) or both (11%).17 Start of therapy was 8.8±9.7 days. None of the ‘other’ tICrH group had hematoma expansion. Five of the SDH (9.1%) had clinically significant expansion; however, only one of these was on an anticoagulant and one on both antiplatelet and anticoagulant (the others were on only antiplatelet therapy). Finally, there is an analysis that suggests a more aggressive approach to restarting.3 Hawryluk et al extracted data from 63 case reports and series on 492 anticoagulated patients with central nervous system hemorrhage finding a rebleeding rate of 8% and a thromboembolic event rate of 6%.3 Temporal mapping (see figure 7) showed most rebleeding events occurred before 72 hours, while most thromboembolic events occurred from 3 days to 1 week, suggesting 3 days would be the ideal restart interval.3 There are several limitations. The analysis is from the warfarin era, only 6.5% of the cases were traumatic, and it is confounded by heterogeneous case report/series reporting bias.
Timing of anticoagulant therapy after severe traumatic intracranial hemorrhage. Reproduced with permission from Divito et al.10 Copyright 2019 Elsevier.
Timing of secondary events following intracranial hemorrhage by event type. Reproduced with permission from Hawryluk et al.3 Copyright 2010 John Wiley and Sons. CNS, central nervous system.
Vitamin K antagonists versus direct oral anticoagulants
VKA such as warfarin had been the standard of care for decades until the DOACs were approved starting in 2010. With simple dosing, no routine monitoring, and less food and drug interactions, DOACs are rapidly replacing warfarin in non-valvular AF and venous thromboembolism treatment and prevention.40 DOACs are also associated with a lower ICrH risk. A possible mechanism involves factor VII. Warfarin inhibits factor VII synthesis (along with factors II, IX, X and proteins C and S) by blocking the enzyme vitamin K epoxide reductase. DOACs target factors IIa and Xa, leaving factor VII intact. The brain is rich in tissue factor, an important protective mechanism in an environment where uncontrolled bleeding is fatal.41 The interaction of activated factor VII with tissue factor causing a thrombin burst is an important hemostatic mechanism as it is the initiation step in both the classic (extrinsic pathway) and cell-based models of coagulation.42 Lower ICrH risk was seen in all the clinical trials of VKA versus DOACs both in AF and VTE treatment populations.43 44 The patient populations that are an exception are those with mechanical valves and ventricular assist devices (VADs). Clinical trials of DOACs in valve patients failed.45 VKAs are still the standard of care in these patients. The prospective trials in restart and timing are primarily testing DOACs and excluding patients with mechanical valves and VADs. A retrospective analysis of mechanical valve patients with ICrH using a composite outcome found the balance between bleeding and thrombosis was best achieved in valve patients at 6.7 days to restart.46 In VAD patients, restarting at 10 days may strike the best balance.47
When extrapolating warfarin data to DOACs, it important to remember that warfarin anticoagulation onset is delayed 5 days or more, and it is not standard to bridge with heparins except in VTE treatment. The restart and timing of restart literature contains primarily warfarin patients as retrospective databases used stretched back before the DOAC era.
Restart following surgical intervention of tICrH
tICrH also frequently requires emergent surgical intervention. These patients are already at elevated risk of hematoma expansion as well as subsequent thromboembolic complications.48 The decision to perform neurosurgical interventions may further alter the subsequent risk profile of recurrent hemorrhage for anticoagulated tICrH. With regard to pre-injury treatment, anticoagulation increases the risks of surgical intervention. In the near term, pre-injury anticoagulant or antiplatelet treatment was not associated with acute, postoperative hemorrhage in a retrospective study of 143 neurosurgical (craniotomy and craniectomy) patients.49 VKA relative to DOAC, antiplatelets or controls has been associated with greater reversal agent use, hematoma expansion and mortality, but unrelated to the need for surgical intervention.50 Another study demonstrated that DOAC treatment pre-injury was associated with greater likelihood of neurosurgical intervention.51 However, a larger study of chronic SDH evacuation demonstrated that cases on preoperative anticoagulation therapy were more likely to experience major hemorrhage during follow-up (OR=1.93, p=0.014), and the time to occurrence of either hemorrhagic or thromboembolic complications was shorter.52
With regard to reinitiation of anticoagulation therapy, the use of DOAC therapy or heparin has been recommended over VKA,48 based on evidence of lower risk of ICrH in non-bleeding populations. There is a real scarcity of evidence for any relationship between surgical treatment for tICrH and the decision to restart anticoagulation. Here as well, selection bias is an obvious concern with any retrospective, observational research into the question of restart. This may partly explain associations found between reinitiation and reduced risk of hemorrhage in unadjusted analyses.49 52 In cases with elevated risk of thromboembolic events, reinitiation as early as 3 days postinjury has also been suggested.49 53 However, the overall strategy for how to manage anticoagulant-associated ICrH after surgery is unclear.16 48 52
Prospective randomized trials
There are several, large clinical trials in preparation or underway which seek to answer questions about restart of anticoagulation treatment following various models of ICrH (see table 1). Most of these trials are focused on answering the question of whether anticoagulation is safe to resume after various types of spontaneous hemorrhage, by comparing anticoagulation with more conservative approaches at varying intervals. These trials and several more investigating antiplatelet therapies have been organized under the Collaboration Of Controlled Randomised trials of Oral Antithrombotic drugs after intraCranial Haemorrhage. The eligibility criteria, intervention arms, time windows after hemorrhage for enrollment, follow-up periods and outcome measures all vary considerably (table 1). However, the primary outcome measure in each instance is a rate based on some composite of secondary clinical events. All studies of spontaneous ICrH employ a usual care or antiplatelet control group.
Ongoing and pending clinical trials of anticoagulation restart after intracranial hemorrhage
One trial (table 1) addresses the question of anticoagulation effectiveness in a mixture of sICrH and tICrH cases (traumatic SDH-only) and one is focused solely on addressing the timing of anticoagulation restart in tICrH (RESTART-tICrH). None specifically exclude neurosurgical intervention.
Spontaneous ICrH only
The SoStart study (University of Edinburgh, NCT03153150) is enrolling all sICrH, including intracerebral, SAH, IVH and (spontaneous) SDH with high-risk non-valvular AF, and randomizing patients to anticoagulation (DOAC or VKA) versus no-anticoagulation (antiplatelet or no antithrombotic therapy). The ASPIRE study (Yale University and National Institute of Neurological Disorders and Stroke (NINDS), NCT03968393) is enrolling patients with ICH with deep and low-risk lobar hemorrhages and randomizing participants to apixaban versus aspirin. STATICH (Oslo University Hospital, NCT03186729) is a trial based out of Norway, enrolling sICH with an indication for antithrombotic therapy and stratified to randomize high-risk (AF history) cases to receive anticoagulant treatment or not. The trial primarily aims to identify the 2-year event rate for recurrent, symptomatic ICH following treatment. Conversely, the A3ICH trial (University Hospital, Lille, NCT03243175) has broad composite outcome definitions, incorporating system-wide, hemorrhagic or ischemic, cardiovascular and cerebrovascular events. A3ICH enrolls sICH cases with high-risk non-valvular AF, comparing treatment between three treatment arms (1:1:1): DOAC treatment (apixaban), left atrial appendage closure or neither intervention. The smallest trial, APACHE-AF trial (University Medical Center Utrecht, NCT02565693) is enrolling spontaneous ICH cases on anticoagulation treatment prior to injury and randomizing them to apixaban or no anticoagulant therapy (antiplatelets or no antithrombotic therapy).
Combined tICrH and sICH eligibility (ENRICH-AF)
The most inclusive study, ENRICH-AF (Population Health Research Institute, NCT03950076), is enrolling all spontaneous ICH associated with anticoagulation treatment for AF, including traumatic and spontaneous SDH, comparing usual care with edoxaban. It also has the broadest time window and includes even patients with a history of ICrH who are not currently anticoagulated. A substudy of ENRICH-AF, designed, implemented and overseen through a partnership between RESTART-tICrH and ENRICH-AF clinical leadership, will examine timing, randomizing 2 vs 4 weeks, in enrolled patients with acute ICrH who randomize to anticoagulation.
Traumatic ICrH only (RESTART-tICrH)
The RESTART-tICrH study (University of Texas, Austin, Texas, NCT04229758) is the only trial specifically focused exclusively on tICrH associated with treatment for AF or VTE and on the timing of treatment reinitiation, randomizing to treatment at 1, 2 or 4 weeks. RESTART-tICrH will compare timing intervals using a composite outcome of secondary hemorrhagic and thromboembolic clinical events. This trial has several unique features, including a pragmatic inclusion strategy, the reliance on a time-as-dose exposure and a response-adaptive randomization mechanism that increases trial efficiency and maximizes allocation to the best performing arm.
Discussion
Clinical equipoise in treatment timing after tICrH, and therefore uncertainty in best course of action, is distributed within the range of current guidelines. Restarting oral anticoagulants after most cases tICrH appears to be supported by broad clinical consensus, but clinical equipoise still exists for the timing, although most clinicians and experts surveyed state they prefer to restart in the first month after injury. The demonstrated existence of competing risks for hemorrhage and thrombosis suggests that there is an optimal timing that can be identified within the time windows offered by current consensus. It is also likely that this timing varies by individual cases’ presentation and risk factors. Many of the observational studies described in this review excluded cases with contraindications for anticoagulation such as hematoma expansion on follow-up imaging. Ongoing and planned clinical trials will shed more light on these important clinical questions. The results of these clinical trials offer unique insights, based on the differences in sample criteria and timing of interventions offered.
References
Footnotes
Contributors Author contributions (CRediT statement): BK, PhD MPH: conceptualization, methodology, writing—original draft; TM Jr, MD: conceptualization, writing—original draft; BG, PhD: conceptualization, writing—review and editing; TWC, MD: conceptualization, writing—review and editing; JW, PhD: writing—review and editing; MAP, PhD: conceptualization, writing—review and editing; DM, PhD: project administration, writing—review and editing; DMS, MD: writing—review and editing; SC, MD: conceptualization, resources, writing—review and editing; AV, MD: conceptualization, writing—review and editing; SW, MD: resources, writing—review and editing.
Funding TM’s effort was supported by grant support from the National Heart Lung and Blood Institute (NHLBI, 1K23HL127227-01A1).
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.