Article Text
Abstract
Background Approximately one-third of musculoskeletal injuries are simple stable injuries (SSIs). Direct discharge (DD) from the emergency department (ED) of patients with SSIs reduces healthcare utilization, without compromising patient outcome and experience, when compared with “traditional” care with routine follow-up. This study aimed to determine the cost-effectiveness of DD compared with traditional care from a societal perspective.
Methods Societal costs, including healthcare, work absenteeism, and travel costs, were calculated for patients with an SSI, 6 months before (pre-DD cohort) and after implementation of DD (DD cohort). The pre-DD cohort was treated according to local protocols. The DD cohort was treated using orthoses, discharge leaflet, smartphone application, and telephone helpline, without scheduling routine follow-up. Effect measures included generic health-related quality of life (HR-QoL; EuroQol Five-Dimensional Questionnaire); disease-specific HR-QoL (functional outcome, different validated questionnaires, converted to 0–100 scale); treatment satisfaction (Visual Analog Scale (VAS), 1–10); and pain (VAS, 1–10). All data were assessed using a 3-month postinjury survey and electronic patient records. Incremental cost-effectiveness ratios were calculated and uncertainty was assessed using bootstrapping techniques.
Results Before DD, 144 of 348 participants completed the survey versus 153 of 371 patients thereafter. There were no statistically significant differences between the pre-DD cohort and the DD cohort for generic HR-QoL (0.03; 95% CI −0.01 to 0.08), disease-specific HR-QoL (4.4; 95% CI −1.1 to 9.9), pain (0.08; 95% CI −0.37 to 0.52) and treatment satisfaction (−0.16; 95% CI −0.53 to 0.21). Total societal costs were lowest in the DD cohort (−€822; 95% CI −€1719 to −€67), including healthcare costs (−€168; 95% CI −€205 to −€131) and absenteeism costs (−€645; 95% CI −€1535 to €100). The probability of DD being cost-effective was 0.98 at a willingness-to-pay of €0 for all effect measures, remaining high with increasing willingness-to-pay for generic HR-QoL, disease-specific HR-QoL, and pain, and decreasing with increasing willingness-to-pay for treatment satisfaction.
Discussion DD from the ED of patients with SSI seems cost-effective from a societal perspective. Future studies should test generalizability in other healthcare systems and strengthen findings in larger injury-specific cohorts.
Level of evidence II.
- fracture
- patient satisfaction
- cost-benefit analysis
- efficiency
Data availability statement
Data are available upon reasonable request. All data are available upon reasonable request to the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
In the Netherlands, 659 000 of 2 million annual emergency department (ED) visits concern musculoskeletal injuries, such as fractures (43%), soft tissue injuries (15%), distortions (5%), dislocations (4%), and tendon injuries (3%).1 Their economic impact is substantial. That is, in the Netherlands, total annual direct healthcare costs and lost productivity costs after injuries are estimated at €2.4 billion and €1.4 billion, respectively.1 Epidemiological studies show that the incidence of injuries is increasing.2 To illustrate, for example, for extremity fractures in the Netherlands, in 2004 the total number was 129 188 versus 170 673 in 2010 and 260 800 in 2019.1 2 A change in healthcare capacity and resources is warranted in anticipation of a further increasing demand and to maintain high-quality trauma care.2
Traditionally, majority of patients with musculoskeletal injuries are reviewed in a fracture clinic within 1 week after initial ED assessment and at regular fixed periods in the weeks thereafter. Consequently, these clinics are characterized by the routine attendance of large numbers of unselected patients who often have relatively minor injuries with excellent prognosis and do not require intervention.3 4
Recent studies indicate that a large proportion of musculoskeletal injuries are simple stable injuries (SSIs) that require reassurance and information, but not routine review.3 4 There is growing evidence that suggests that direct discharge (DD) from the ED of patients with these SSIs is a safe and effective alternative to “traditional” care with routine follow-up, which reduces healthcare utilization (ie, hospital visits and imaging), without compromising patient outcome (eg, functional outcome) and experience (eg, satisfaction with treatment).5–24
Although a relatively small number of previous studies included cost estimations before and after DD,7–11 to our knowledge currently no studies have been performed regarding the cost-effectiveness. Therefore, the aim of this study was to determine whether DD from the ED of patients with SSIs is cost-effective from a societal perspective compared with “traditional” care with routine follow-up.
Methods
Design
This cost-effectiveness analysis (CEA) was based on data from a prospective non-inferiority trial for 11 SSIs performed in the OLVG Hospital, a level 2 trauma center and teaching hospital in Amsterdam, the Netherlands.25
Participants
All consecutive ED patients with an isolated SSI were asked to participate during a 1-year period (November 15, 2018–November 15, 2019).25 DD was implemented at the midpoint (May 20, 2019), dividing participants into the pre-DD cohort and the DD cohort. Exclusion criteria were inability to understand/complete Dutch survey; initial treatment in the ED of a different hospital; other reasons for follow-up (eg, social care reasons); eye/motor/verbal score <15 at presentation and high-energy trauma; treatment continued elsewhere (eg, closer to home); and alcohol/drug intoxication.25
All patients who were willing to participate provided written informed consent in the ED and received a 3-month postinjury survey containing questions regarding employment status, education level, health-related quality of life (HR-QoL), functional outcome, satisfaction, pain, primary healthcare use, and work absenteeism. Furthermore, the electronic patient record of all participants was evaluated after 1 year to assess secondary healthcare use, including hospital visits, imaging (eg, X-ray, CT scan, MRI scan) and adverse outcomes.
Treatment
Traditional care
Before DD, all patients with SSI were treated using local protocols. This usually involved application of a cast/splint/bandage and review in the fracture clinic 1 week after injury. Frequently, one or multiple subsequent visits followed in the weeks thereafter for assessment of functional outcome and/or removal of cast material.
Direct discharge
The implementation of DD standardized the immobilization of SSIs to bandage or removable orthoses (table 1). Follow-up appointments are no longer scheduled routinely. Instead, ED physicians provide information about the SSI and the expected recovery, which is also summarized in discharge leaflets. These leaflets are available on paper as well as digitally by means of a smartphone application for iOS and Android, which also contains videos of physical exercises to improve recovery and videos explaining how to reapply immobilization after removal (eg, after taking a shower). Patients are instructed to contact our telephone helpline in case of questions or concerns. The X-rays of all ED patients, including patients with SSIs, are reviewed daily by an orthopedic trauma surgeon and a radiologist. If based on this review an injury is not deemed suitable for DD (eg, the injury is not an SSI), a face-to-face appointment is scheduled.
Simple and stable injuries, criteria, and immobilization
Effect measures
Cost-effectiveness was assessed for four outcomes: (1) generic HR-QoL, (2) disease-specific HR-QoL, (3) satisfaction with treatment, and (4) pain. Questionnaires used to assess generic and disease-specific HR-QoL were only available for patients ≥4 years old. Therefore, these outcomes were not assessed for participants <4 years old.
Generic HQ-QoL was assessed using the EuroQol Five-Dimensional Questionnaire (EQ-5D). The five-level version (EQ-5D-5L) was completed by all participants ≥18 years old and also by children ≥12 years old if self-completing the questionnaire.26 The youth version (EQ-5D-Y) was used for all other children 4 to 17 years old.27 Proxy versions were used if a parent/caregiver completed the survey. The participants’ EQ-5D-5L or EQ-5D-Y health states were converted to utility values using the Dutch EQ-5D-5L and EQ-5D-3L value sets, respectively.28 29 Utility values indicate the preference or desirability of a certain health state on a scale anchored at 0 (equal to death) to 1 (equal to optimal health).
Disease-specific HR-QoL was assessed in participants ≥4 years old using four different validated functional outcome questionnaires based on age and type of injury (online supplemental appendix A): (1) shortened version of the Disabilities of the Arm, Shoulder and Hand (QuickDASH),30 (2) Lower Extremity Functional Scale,31 (3) Short Form of the Patient-Reported Outcomes Measurement Information System (PROMIS) Upper Extremity, and (4) Short Form of the PROMIS Mobility.32 33 To compare disease-specific HR-QoL for all participants, the individual functional outcome scores were converted to a single scale ranging from 0 (worst functional outcome) to 100 (best functional outcome).34
Supplemental material
Satisfaction with treatment was assessed using a Visual Analog Scale (VAS), ranging from 0 mm (very dissatisfied) to 100 mm (very satisfied).25 Pain was assessed on a VAS from 0 mm (no pain) to 100 mm (extremely painful). Both scores were converted from a 0 to 100 scale to a 0 to 10 scale.
Cost measures
Costs were assessed from a societal perspective, including healthcare costs, work absenteeism costs, and travel costs.
Healthcare costs included primary healthcare costs (eg, visits to the general practitioner (GP) or physiotherapist for follow-up of SSI) and secondary healthcare costs (eg, ED visits, immobilization materials used, follow-up appointments, no-shows, and imaging).35 Healthcare costs were valued using Dutch standard costs, and if unavailable, prices from professional organizations and hospital accounting records (online supplemental appendix B).
Supplemental material
Work absenteeism costs were based on patient absenteeism and parent absenteeism. Patient absenteeism was estimated using the number of days patients had to take leave from work after sustaining their injury, as well as the number of hours patients had to take leave from work to attend their fracture clinic appointment(s). Parent absenteeism costs were estimated using the number of hours parents had to take time off from work to accompany their child during a hospital visit. For patient absenteeism, costs were calculated using the friction cost approach (FCA) and gender-specific price weights. The FCA assumes that costs are limited to the friction period, that is, the period needed to replace a sick worker, which is 12 weeks in the Netherlands. If applicable, absenteeism costs were therefore truncated at 12 weeks. In case of parent absenteeism, the accompanying parent’s gender was unknown, and consequently gender-specific price weights could not be used. Therefore, these costs were calculated using the average productivity costs rate of all Dutch adults.35
Travel costs per hospital visit were assessed using previously collected hospital survey data on the type of transport and the average distance from a patient’s home to the nearest hospital in the Netherlands and valued using Dutch standard prices (online supplemental appendix B).35
All costs were converted to Euros 2019 using Dutch consumer price indices. Discounting of costs was not necessary because follow-up was not more than 12 months.35–39
The following baseline characteristics were assessed: age, gender, education level (low/middle/high),40 school/work status, and type of injury.
Statistical analysis
Statistical analysis was performed using SPSS V.27.0 (IBM, NY, USA) and STATA V.16 (StataCorp, TX, USA).41 42 As children <4 years old did not receive EQ-5D and functional outcome questionnaires, analyses were performed for two groups separately. That is, the CEAs for satisfaction with treatment and pain were based on data of all participants, whereas the CEAs for generic HR-QoL and disease-specific HR-QoL were based on data of participants ≥4 years old only.
Aggregate and disaggregate cost differences were estimated using linear regression analyses adjusted for patients’ propensity score and work status (yes/no). A propensity score indicates the probability of a patient being assigned to an intervention group given a set of baseline characteristics.43 In our study, the propensity score was estimated using cohort (pre-DD cohort/DD cohort), age, gender, and injury type, using the pscore package in STATA.
Seemingly unrelated regression (SUR) analyses were performed to estimate the differences in costs (∆C) and effects (∆E). Cost differences were adjusted for propensity score and work status, whereas effect differences were adjusted for propensity score. Incremental cost-effectiveness ratios (ICERs) were calculated by dividing the differences in total societal costs by the differences in effects (ie, ∆C/∆E). The 95% CIs around all cost differences were estimated using bias-corrected and accelerated (BCA) bootstrapping, with 5000 replications. BCA bootstrapping was also used to graphically illustrate the uncertainty surrounding the ICER by plotting bootstrapped incremental cost–effect pairs (CE pairs) on cost-effectiveness planes (CE planes).44 45 A summary measure of the joint uncertainty of costs and effects was presented using cost-effectiveness acceptability curves (CEACs), providing an indication of the probability of DD being cost-effective in comparison with traditional care at different values of willingness-to-pay (WTP). WTP is defined as the maximum amount of money decision makers are willing to pay per one extra unit of effect.46 For the differences in costs and effects, a p value of <0.05 was considered statistically significant.
Sensitivity analyses
Three sensitivity analyses were performed to test the robustness of the results of our main analyses: (1) applying the healthcare perspective (SA1), (2) absenteeism costs valued using both age-specific and gender-specific price weights (SA2), and (3) using an alternative propensity score, which was estimated using cohort, age, gender, injury type, and education level (SA3). Both SA2 and SA3 were adjusted for the same covariates as the main analysis, that is, propensity score and work status, whereas SA1 was adjusted for propensity score only.
Patient and public involvement
Patients were not involved in the design, intervention, research question, or outcome measures of the current study.
Results
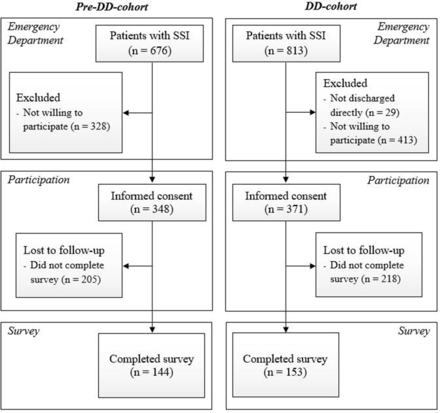
Before DD, 348 of 676 ED patients with SSIs were willing to participate (pre-DD cohort) versus 371 of 784 patients thereafter (DD cohort). Of these, 144 and 153 participants completed the 3-month postinjury survey in the pre-DD cohort and the DD cohort, respectively (figure 1, table 2). The median age in the pre-DD cohort was 26 versus 36 in the DD cohort. There were more employed participants in the DD cohort (47.7%) compared with the pre-DD cohort (40.3%). There were no missing data.
Baseline characteristics
Flow chart depicting the inclusion of patients. In the DD cohort, patients in the emergency department with SSI were used to assess implementation. Patients who provided informed consent were used to assess healthcare utilization, and patients who completed the survey were used to assess patient-reported outcomes and experiences. DD, direct discharge; SSI, simple stable injury.
Effect measures
There were no statistically significant effect differences between the pre-DD cohort and the DD cohort for generic HR-QoL, disease-specific HR-QoL, satisfaction with treatment, and pain (table 3).
Differences in costs and effects, incremental cost-effectiveness ratios, and cost-effectiveness planes
Cost measures
On average, total societal costs were €2181 in the pre-DD cohort versus €1672 in the DD cohort. After adjusting for propensity score and work status, costs were €822 lower in the DD cohort compared with the pre-DD cohort (95% CI −€1719 to €67; table 4). As for the disaggregate cost differences, there were statistically significant reductions in total healthcare costs (−€168), secondary healthcare costs (−€152), parent absenteeism (−€47), and travel costs (−€7) in the DD cohort compared with the pre-DD cohort, whereas there were non-significant reductions in primary healthcare costs (−€16), total absenteeism costs (−€645), and patient absenteeism costs (−€598).
Mean costs and mean cost differences in euro
Cost-effectiveness
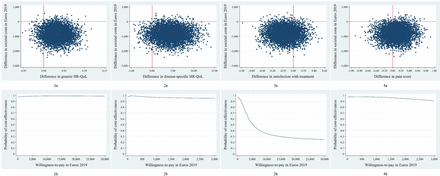
The main analyses for generic HR-QoL indicated that total societal costs were lower in the DD cohort compared with the pre-DD cohort (mean −€845; 95% CI −€1781 to −€88). The ICER was −26 022, indicating that, on average, a one-point improvement in utility in the DD cohort was associated with a societal cost saving of €26 022 compared with the pre-DD cohort. Majority of the the CE pairs were located in the southeast quadrant (87%), indicating that, on average, DD was less costly while being more effective than traditional care (table 3, figure 2-1a).47 The CEAC in figure 2-1b indicates that the probability of DD being cost-effective compared with traditional care is 0.98 at a WTP of 0€/one-point improvement, increasing to a maximum of 0.99 for higher values of WTP.
Cost-effectiveness. (a) Cost-effectiveness planes indicating the distribution of incremental cost–effect pairs around its four quadrants and (b) cost-effectiveness acceptability curves indicating the probability of direct discharge being cost-effective in comparison with standard care for different values (€) of willingness-to-pay for (1) generic HR-QoL, (2) disease-specific HR-QoL, (3) pain, and (4) treatment satisfaction. HR-QoL, health-related quality of life.
For disease-specific HR-QoL, the ICER was −193, and 88% of CE pairs were located in the southeast quadrant, indicating that DD dominated traditional care (ie, less costly and more effective than traditional care (table 3, figure 2-2a). The probability of DD being cost-effective was 0.98 at a WTP of 0€/one-point improvement on the 1 to 100 functional outcome scale, slightly decreasing to 0.95 at a WTP of €3000 per point improvement (figure 2-2b).
The ICER for satisfaction with treatment was 5213, suggesting that, on average, a one-point decrease on the 1 to 10 VAS was associated with a societal cost saving of €5213 (table 3). Majority of the CE pairs (74%) were located in the southwest quadrant, indicating that, on average, DD was less costly and less effective than traditional care (figure 2-3a). The CEAC indicates that the probability of DD being cost-effective compared with traditional care was 0.98 at a WTP of 0€/one-point improvement, decreasing with increasing values of WTP (0.92 at €1000, 0.69 at €3000, and a minimum of 0.23 at €30 000; figure 2-3b).
The ICER for pain was −10 517, and 61% of CE pairs were located in the southeast quadrant, suggesting that, on average, DD dominated traditional care (table 3, figure 2-4a). At a WTP of 0€/one-point improvement on the 1 to 10 VAS, the probability of DD being cost-effective compared with traditional care was 0.98 (figure 2-4b). This probability slightly decreased with increasing values of WTP to 0.91 at a WTP of €3000.
Sensitivity analyses
In accordance with the main analysis, total costs were statistically significantly lower in the DD cohort compared with the pre-DD cohort when applying the healthcare perspective (SA1), but the magnitude of this effect was smaller. When absenteeism costs were valued using age-specific and gender-specific price weights (SA2), and when an alternative propensity score was used (SA3), results regarding costs, effects, ICERs, and CEACs were comparable with those in the main analysis, although differences in costs were no longer statistically significant.
Discussion
The results of this study suggest that DD from the ED of patients with SSIs is likely to be cost-effective compared with “traditional” care with routine follow-up, both from a societal perspective as well as a healthcare perspective, for all four effect measures. That is, DD dominates traditional care for generic HR-QoL, disease-specific HR-QoL, and pain; that is, for these outcomes, on average, DD was less costly and more effective. For satisfaction with treatment, on average, DD was less costly, but also less effective compared with traditional care. It is noteworthy, however, that the mean difference in satisfaction with treatment was 0.16 on a 0 to 10 scale in favor of the pre-DD cohort,25 which is far from clinically relevant as the minimally clinical important difference for this outcome is estimated at 0.7. Furthermore, in both cohorts there were relatively high levels of satisfaction; for example, the mean satisfaction with treatment in the DD cohort was 7.95.25
For all outcomes, the CEACs indicate that DD has a high probability of being cost-effective compared with traditional care. For generic HR-QoL, disease-specific HR-QoL, and pain, these probabilities remained the same or only slightly decreased with increasing values of WTP. For satisfaction with treatment, this probability slightly decreased with reasonable increasing values of WTP. This decrease in WTP was caused by the fact that satisfaction with treatment was, on average, lower for DD compared with traditional care, but as mentioned this difference was far from clinically relevant.
Although the aim of this study was to assess cost-effectiveness, our results also suggest that DD is likely to reduce costs across all included cost categories, that is, absenteeism, primary healthcare, secondary healthcare, and travel costs. Based on previous studies, we estimate that approximately 85 000 annual ED visits concern SSIs,1 3 4 and based on the results of the current study, if DD would become the standard of care in the Netherlands, this could potentially result in a national societal cost saving of €70 million per year. This includes an annual reduction of €14 million in direct healthcare costs and €54.8 million lost productivity costs. Although this is a relatively small proportion of the total annual societal costs of injuries (€3.8 billion),1 it is also important to consider the additional logistic benefits of DD; that is, in our study on effectiveness of DD, we estimated that national adoption of DD would prevent approximately 142 800 outpatient visit clinics per year.25
Several previous studies on DD of SSIs have attempted to quantify healthcare cost differences before and after DD, including several non-comparative studies that modeled pre-DD costs based on several assumptions with regard to healthcare utilization, rather than actually measuring pre-DD data.10 11 18 Furthermore, several previous comparative studies only assessed healthcare costs, most frequently limited to secondary healthcare costs, and did not assess cost-effectiveness.7–9 To our knowledge, the current study is the first to assess costs and cost-effectiveness from the broader societal perspective, including both primary healthcare costs and secondary healthcare costs, as well as work absenteeism costs and travel costs. The societal perspective is recommended by the Dutch guidelines for economic evaluations in healthcare.48
As a result of this, the comparison of our results with previous studies is limited to comparing healthcare cost differences. It must be noted, however, that the ability to directly compare these differences is further limited by the differences in healthcare (payment) systems across countries as well as the variety of variables used to calculate the cost differences and the costs per item. To illustrate, Seewoonarain et al9 (Great Britain) valued one fracture clinic appointment at £154, whereas Mackenzie et al8 valued this at £99 (Scotland), versus €85 in the current study based on Dutch reference prices (online supplemental appendix B). Moreover, in our study, on average, primary and secondary healthcare costs were reduced by €16 and €152 per patient, respectively. Hamilton et al7 reported a mean reduction in healthcare costs of £100 (€116) after DD in a comparative study among pediatric patients with a forearm fracture. In this study, however, healthcare costs only included clinic visit, GP visit, and immobilization material costs. Seewoonarain et al9 compared patients with a torus fracture of the distal radius before and after DD and found a mean reduction of £62 (€72) in healthcare costs per patient after DD. Their costs only included clinic costs and immobilization costs, which might explain why their total cost reduction was smaller than ours.9 Mackenzie et al8 estimated the secondary healthcare costs of three types of SSIs before and after DD. Costs included staffing costs, operation costs, and radiology costs. The median reduction per patient was £128 (€148) for fifth metacarpal fractures, £84 (€97) for fifth metatarsal fractures, and £138 (€160) for radial head fractures.8
This study has several strengths. Most importantly, this is the first study to evaluate the cost-effectiveness of DD compared with traditional care from a societal perspective. Second, cost-effectiveness was analyzed using SUR. This regression technique allows for the correction of the possible correlations between costs and effects.49 Third, this study was based on an extensive survey, which allowed a wide range of variables to be included in our total societal cost estimates. Fourth, although randomization might be infeasible for studying redesigns such as DD, our study deals with the non-randomized nature of the study using propensity score adjustments. Last, there were no missing data among the participants who responded to the survey.
This study also has several limitations. First, both cohorts were relatively small, and consequently the 95% CIs surrounding the cost-differences are relatively wide. However, we do not expect our analyses to be extremely underpowered because even our total cost differences were found to be statistically significant, whereas costs are typically underpowered due to their relatively skewed nature. Second, however in keeping with what might be expected, the survey response rate was relatively low, but there were no major differences in baseline characteristics between responding and non-responding participants.25 Third, 11 types of SSIs were included and this limits the ability to draw injury-specific conclusions regarding (cost-)effectiveness of DD versus traditional care. Preliminary post-hoc analyses per injury are provided in online supplemental appendix C for societal costs, healthcare costs, and the four effect measures that were used in our study. These analyses indicate that DD reduces both societal costs as well as healthcare costs for all injury subgroups, whereas there were no remarkable differences in patient outcomes or patient experiences before and after implementing DD for each injury. However, these injury subgroups are relatively small and therefore lack statistical power; hence, future studies should be conducted to assess this in more detail. Fourth, in this CEA we did not consider the one-time investment that is required to implement DD in a hospital (eg, staffing, resources). However, we have shared our protocols and discharge leaflets with other Dutch hospitals, and these hospitals typically require approximately 3 months to prepare the implementation. Therefore, the associated costs for preparing implementation are relatively low and in our opinion should not refrain hospitals from implementing this model of care. Fifth, outcomes were only measured at a single point in time. Hence, we were unable to include quality-adjusted life years as outcome measure. Sixth, most data were collected prospectively by self-report. Although we do not expect this to differ severely between cohorts, this may have caused socially desirable answers and/or recall bias, for example, for number of GP visits or work absenteeism. Last, patient absenteeism was valued using age-specific and gender-specific price weights, but parent absenteeism was valued using average wage, as the gender and age of the parent accompanying the child during follow-up were unknown.
Supplemental material
Future studies should focus on the generalizability of our results in other countries with different healthcare systems, as well as the eligibility of other injuries for DD and associated (cost-)effectiveness. Additionally, studies including larger cohorts should be conducted to strengthen the results, and these cohorts should be large enough to perform subgroup analysis for each injury. Currently multiple hospitals in the Netherlands are implementing DD and therefore this might be achieved by performing multicenter studies in which data are collected in a cooperative and standardized manner. Last, researchers could explore the options to shift the treatment of patients with SSIs from secondary healthcare to primary healthcare (eg, treatment via GP without visiting the ED at all) to further reduce healthcare and societal costs.
In conclusion, the results of this first CEA to compare DD of SSIs with traditional care suggest that DD is likely to be cost-effective compared with “traditional” care with routine follow-up, for generic-specific and disease-specific HR-QoL, satisfaction with treatment, and pain, both from a societal perspective as well as from a healthcare perspective. As a large proportion of musculoskeletal injuries are SSIs, the implementation of DD offers an opportunity to respond to increasing demands, high workloads, and costs. This is necessary to guarantee future high-quality trauma care. Future studies should be performed in larger cohorts to strengthen current findings as well as to test generalizability in other countries.
Data availability statement
Data are available upon reasonable request. All data are available upon reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethical committee of the OLVG Hospital (ref no 18.071) and by its Board of Directors.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
THG and NJG are joint first authors.
Collaborators Virtual Fracture Care Study Collaborative: Bas A Uijterwijk (Msc, resident surgery) and Ruth J Stoffels (Msc, data analyst) contributed to the collection of data for this study.
Contributors THG was responsible for the conduct of this study, the methodology, draft of the article, collection of data, and analysis of all data. NJG was responsible for the draft of the article and analysis of all data. JMvD contributed to the methodology, writing of the article, and analysis of all data. RH, RNvV and JCG all contributed to the methodology of this study, implementation of direct discharge, as well as the writing of the article. JCG was the guarantor.
Funding This work was supported by an unrestricted grant provided by Zilveren Kruis Achmea (Leiden, The Netherlands) (grant ID number: WN-2019-060).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.