Article Text
Abstract
Background There is a critical need for non-narcotic analgesic adjuncts in the treatment of thoracic pain. We evaluated the efficacy of intercostal cryoneurolysis as an analgesic adjunct for chest wall pain, specifically addressing the applicability of intercostal cryoneurolysis for pain control after chest wall trauma.
Methods A systematic review was performed through searches of PubMed, EMBASE, and the Cochrane Library. We included studies involving patients of all ages that evaluated the efficacy of intercostal cryoneurolysis as a pain adjunct for chest wall pathology. Quantitative and qualitative synthesis was performed.
Results Twenty-three studies including 570 patients undergoing cryoneurolysis met eligibility criteria for quantitative analysis. Five subgroups of patients treated with intercostal cryoneurolysis were identified: pectus excavatum (nine studies); thoracotomy (eight studies); post-thoracotomy pain syndrome (three studies); malignant chest wall pain (two studies); and traumatic rib fractures (one study). There is overall low-quality evidence supporting intercostal cryoneurolysis as an analgesic adjunct for chest wall pain. A majority of studies demonstrated decreased inpatient narcotic use with intercostal cryoneurolysis compared with conventional pain modalities. Intercostal cryoneurolysis may also lead to decreased hospital length of stay. The procedure did not definitively increase operative time, and risk of complications was low.
Conclusions Given the favorable risk-to-benefit profile, both percutaneous and thoracoscopic intercostal cryoneurolysis may serve as a worthwhile analgesic adjunct in trauma patients with rib fractures who have failed conventional medical management. However, further prospective studies are needed to improve quality of evidence.
Level of evidence Level IV systematic reviews and meta-analyses.
- rib fractures
- thoracotomy
- analgesia
- epidural
Data availability statement
Data are available in a public, open access repository
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Background
A cornerstone of treatment for chest wall pain from surgery or trauma is adequate analgesia due to the associated morbidity and mortality of suboptimal pain control.1 One emerging adjunct for chest wall pain is cryoneurolysis. Cryoneurolysis is the direct application of cold to nerves, resulting in Wallerian degeneration and pain relief for 6–12 months.2 3 Yet, despite its growing utilization, limited data are available regarding the efficacy of intercostal cryoneurolysis (IC) for chest wall pain after surgery or injury. To assess the potential applicability of cryoneurolysis for pain control after chest wall trauma, we performed a systematic review evaluating efficacy of IC as an analgesic adjunct for multiple etiologies of chest wall pain. The goal was to review literature supporting the use of IC for a range of surgical conditions with the intent of providing an evidence base to which we could compare existing studies evaluating IC in traumatically injured patients.
Methods
This was an Institutional Review Board exempt study, as all articles were publicly available.
Registration
The study protocol was submitted to PROSPERO (International Prospective Register of Systematic Reviews) before abstract screening and was registered under ID CRD42020210372.
Study selection
A research librarian performed extensive searches of three bibliographic databases: PubMed (includes MEDLINE), EMBASE, and the Cochrane Library. Eligible articles included experimental and observational studies, excluding case reports, in the English language published from database inception to August 26, 2020. We included studies involving patients of all ages, evaluating efficacy of IC as a pain adjunct for any thoracic wall pathology. We excluded studies evaluating cryosurgery as treatment for any mediastinal pathology involving the esophagus and heart, cryoneurolysis not involving intercostal nerves, or when primary or secondary outcomes did not include pain-related factors. Database search results were uploaded to Covidence, a Cochrane-sanctioned web-based application that facilitates screening reviewing. Two reviewers (PIC and JGM) screened potentially eligible studies using standardized forms. Disagreements were resolved after discussion and consensus with a third reviewer (JDF).
One pair of reviewers (PIC and JGM) independently used standardized data extraction forms. Quality of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework for cohort studies.4 Bias identified in reviewed studies was described in the qualitative assessment.
Results
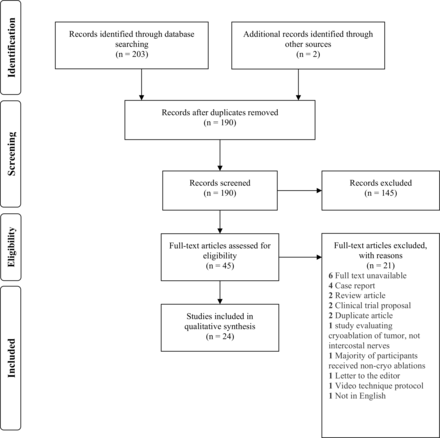
We screened 205 studies for eligibility. Of these, 24 met eligibility criteria for quantitative analysis (figure 1), which comprised 575 patients undergoing cryoneurolysis. The studies were performed between 1974 and 2020 and spanned seven different countries. Five subgroups of patients were identified: pectus excavatum (nine studies); thoracotomy (eight studies); post-thoracotomy pain syndrome (PTPS; three studies); chest wall pain from malignancy (two studies); and traumatic rib fractures (two studies).
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram showing selection process of included articles.
Pectus excavatum
PICO 1: in patients undergoing minimally invasive pectus excavatum repair, does IC compared with conventional analgesia lead to superior pain control and shorter length of hospital stay?
For patients undergoing surgery for pectus excavatum (ie, Nuss or Ravitch procedure), overall quality of evidence was low-moderate with one randomized controlled trial, one prospective cohort, six retrospective cohorts and one descriptive study (table 1). Seven studies evaluated the effect of IC compared with epidural or patient-controlled analgesia (PCA) on hospital length of stay and demonstrated decreased length of stay for patients receiving cryoneurolysis. Five studies showed decreased inpatient narcotic usage when undergoing IC but only three showed improved pain scores at any point after surgery versus control. Only four out of seven studies showed increased operative time with IC. No studies found increased complication rates with IC. All studies were limited in their small sample size.
Pectus excavatum
In 2016, Keller et al5 conducted a retrospective review of 52 children undergoing Nuss procedure with either IC (n=26) or thoracic epidural (n=26) in 2016. Patients who received cryoneurolysis had shorter length of stay (3.5 vs. 5.8 days, p<0.001) and decreased total intravenous narcotic usage (49.0 vs. 119.8 morphine milligram equivalents (MME), p=0.001). However, those receiving cryoneurolysis had longer mean surgery time (167.8 vs. 147.9 minutes, p=0.01). Limitations included short follow-up interval and lack of statistical analysis.
In 2017, Harbaugh et al6 performed a single-center retrospective review of 32 pediatric patients undergoing Nuss procedure comparing cryoneurolysis (n=19) to thoracic epidural (n=13). Those receiving cryoneurolysis had decreased length of stay (3 vs. 6 days, p<0.001). There was no difference in inpatient postoperative opioid use, pain scores, operative time or complication rates. Limitations included lack of longer term follow-up and inability to account for neuraxial opioid administration leading to potential confounding effects.
In 2018, Morikawa et al7 performed a retrospective review comparing children who received cryoneurolysis (n=6) during Nuss repair to those who did not (n=13). Patients who underwent cryoneurolysis were discharged faster (2.2 vs. 3.7 days, p=0.01) and required lower narcotic doses (6.4 vs. 17.9 morphine equivalents (ME), p=0.05). There were no significant differences in highest postoperative pain score, operative time, or complications. Limitations included differences in narcotic regimen between groups.
In 2018, Sujka et al8 conducted a retrospective study evaluating 28 patients undergoing pectus excavatum repair, nine of whom underwent cryoneurolysis and the remaining 19 using a combination of epidural and/or PCA. In the cryoneurolysis group, hospital length of stay (1.4 vs. 4.0 days, p=0.00), days to only oral pain medications (1.2 vs. 2.6 days, p=0.00), and postoperative day 2 pain scores (3.3 vs. 6.1, p=0.03) were less. Operative time was longer (98.4 vs. 64.2 minutes, p=0.00). One patient in the cryoneurolysis group developed pneumothorax but there were no significant differences in complication rates. Limitations included lack of long-term follow-up.
In 2019, Graves et al9 executed a randomized clinical trial of 20 patients undergoing the Nuss procedure who received either cryoneurolysis (n=10) or thoracic epidural (n=10). Patients receiving cryoneurolysis had decreased length of stay (3 vs. 5 days, p=0.0001) and fewer inpatient opioids (mean decrease of 416 mg oral morphine equivalent, p=0.0001). There was no difference in mean pain scores at any point and no complications. Limitations included intrinsic differences between control and treatment groups (mean age 16.1 vs. 20.9 years, p=0.03), which may have confounded results.
In 2019, Pilkington et al10 conducted a retrospective review of pediatric patients undergoing modified Ravitch procedure with either IC (n=9) or thoracic epidural (n=20). Those receiving cryoneurolysis had shorter length of stay (4 vs. 6 days, p=0.006) and lower pain scores by postoperative day 2 (3 vs. 4, p=0.0004). There were no differences in total postoperative opioid usage, operative time or complications. Limitations included inability to include epidural narcotics in analysis and non-standardized non-opioid analgesia regimens.
In 2020, Dekonenko et al11 performed a prospective observational study comparing 35 patients undergoing minimally invasive pectus excavatum repair with IC to patients involved in a randomized controlled trial comparing efficacy of epidurals (n=32) versus PCA (n=33). Cryoneurolysis was associated with decreased length of stay (1 day vs. 4.3 and 4.2 days for epidural and PCA groups, p<0.01), less total inpatient MME required (p<0.01), and longer operative time (101 minutes vs. 58 and 57 minutes, p<0.01). One patient (3%) in the cryoneurolysis group required a chest tube for right-sided pneumothorax. Limitations included lack of follow-up data and limiting assessment on longer term opioid usage and any late postoperative complications.
In 2020, Cadaval Gallardo et al12 performed a retrospective analysis of 21 patients who underwent bilateral thoracoscopic cryoneurolysis of 3rd−7th intercostal nerves during minimally invasive pectus excavatum repair in Sevilla, Spain. Mean patient age was 15.2±4.3 years, average Haller index 5.1±3.0, and mean number of implants 2.6±0.7. Mean postoperative stay was 1.6±0.7 days. Seventy-one percent required 0–1 opioid dose postoperatively. Mean visual analog scale (VAS) pains scores decreased further out from surgery (postoperative day 1: 2.5±1.8, postoperative day 7: 0.5±0.9). No major intraoperative or postoperative complications were noted. Limitations of this study included its retrospective nature.
In 2020, Zobel et al13 performed a retrospective review of both pediatric and adult patients who underwent IC during the Nuss procedure and evaluated for differences in length of stay, neuropathic pain development, and duration of chest numbness between young (≤21 years old) (n=30) and older patients (n=13). Mean length of stay was shorter in the younger group (2 vs. 3.9 days, p=0.03). The older group experienced more neuropathic pain (38.5% vs. 0.0%, p=0.001) and longer duration of chest numbness (10.7 vs. 3.4 months, p=0.003). Limitations included inconsistency in the length of time between surgery and survey administration.
PICO 1 overall recommendation: For pediatric patients undergoing minimally invasive pectus excavatum repair, cryoneurolysis compared with epidural or PCA leads to shorter length of stay without any increased complication rates (GRADE 2A). Cryoneurolysis as an analgesic adjunct leads to decreased inpatient narcotic use but does not significantly impact patient pain scores (GRADE 2B). IC does not definitively lead to increased operative time (GRADE 2C)
Thoracotomy
PICO 2: in patients requiring thoracotomy, does cryoneurolysis compared with conventional analgesia lead to superior pain control and shorter length of hospital stay?
In adult patients undergoing thoracotomy, overall quality of evidence was moderate with four randomized controlled trials, two non-randomized controlled trials, one retrospective cohort, and one descriptive study (table 2). No studies were able to demonstrate decreased length of stay with IC, six of eight showed decreased inpatient narcotic use, and three of four found improved pain scores at any point postoperatively. Only one study evaluated for differences in operative time and complications and found no difference compared with control.
Thoracotomy
In 1974, Nelson et al14 performed a non-randomized controlled trial of 76 individuals. Participants receiving posterolateral and anterior thoracotomies were randomized to either receive cryoneurolysis or not. There was a significantly decreased postoperative narcotic usage in those treated with cryoneurolysis (cryo 661.0 mg vs. no cryo 855.2 mg of meperidine). Patients with IC did not demonstrate neuritis or neuromas in 24 months of follow-up. Limitations of this study included limited sample size and non-randomization of study groups, which may have led to systematic differences between groups affecting outcomes. Three patients had two or more operations and served as their own controls, which may have confounded the data.
In 1980, Glynn et al15 performed a non-randomized controlled trial of 58 adults. Twenty-nine patients undergoing thoracotomies for cancer resection, spontaneous pneumothorax, lung abscess, and hiatal hernia were assigned to the group receiving cryoneurolysis. Twenty-nine matched patients were assigned to the control group that received only postoperative analgesia. Patients who received cryoneurolysis required less narcotics after surgery than those in the control group (number of postoperative injections: 5 vs. 9, p<0.005). There was no significant difference in time to mobilization and time to discharge between the two groups. Limitations included smaller sample size at a single center, non-randomization, and study groups not being matched for type of incision (eg, all four thoracolaparotomies were in the cryoneurolysis group).
In 1980, Katz et al16 performed a randomized controlled trial of 24 individuals with one group receiving cryoneurolysis and the other either receiving intercostal blocks with long-acting local anesthetics or not receiving any block. Cryoneurolysis significantly reduced pain scores early postoperatively. Full return of chest wall sensation occurred in all patients by postoperative day 30, and there were no adverse sequelae 6 months afterwards. Limitations of the study included that the first six patients assigned to the IC group were non-random and essentially learning cases where ‘technique was being refined’.
In 1981, Maiwand and Makey17 performed a descriptive study on 100 patients undergoing cryoneurolysis during thoracotomy for various thoracic etiologies. Seventy-nine were reported to be pain free, 12 experienced discomfort, and 9 had severe pain requiring narcotics. Normal sensation returned after 3–6 months. Limitations for this study included its non-experimental study design, absence of objective, quantitative pain markers, and absence of long-term follow-up.
In 1986, Brynitz and Schrøder18 performed a partially blinded randomized controlled trial on 27 patients undergoing lateral thoracotomy without rib resection. Only the surgeon was informed of each patient’s treatment group. Compared with control, the cryoneurolysis group used significantly less nicomorphine (35.7 vs. 3700 mg, p<0.05). One major limitation of this study was that both study groups received morphine and dextropropoxyphene, which was unaccounted for, likely confounding data on narcotic usage.
In 2000, Bucerius et al19 performed a randomized controlled trial of 57 patients undergoing lateral mini-thoracotomy for mitral valve surgery or minimally invasive direct coronary artery bypass grafting (MIDCABG), of whom 30 were randomly assigned to IC. Postoperative pain scores from postoperative days 1–7, and overall pain levels were found to be lower in the IC group (p<0.0001). In the MIDCABG group who required a more traumatic operative approach, those receiving cryoneurolysis suffered significantly less pain compared with control in the early postoperative period whereas no differences in pain scores were seen in the less invasive mitral group during the early postoperative period. Study limitations included technique deviation as only one intercostal level was ablated and lack of longer term follow-up as pain scores and narcotic requirement were only measured up to 1 week out from surgery.
In 2004, Yang et al20 performed a randomized controlled trial with 90 participants undergoing thoracotomy for malignant disease. Forty-five patients were prospectively randomized to epidural analgesia alone and 45 patients to combined epidural analgesia and cryoneurolysis. Patients in the cryoneurolysis+epidural group suffered less pain by postoperative day 7 (p=0.04) and required a smaller rescue dose of morphine on postoperative day 6 (p=0.04) and day 7 (p=0.02). Incidence (n=15 vs. 6, p=0.04) and severity (numeric rating scale 2.0 vs. 3.0, p=0.01) of PTPS at rest were higher in the cryoneurolysis+epidural group at 3 months but that difference disappeared by 6 months. Limitations of this study included failure to isolate effects of cryoneurolysis itself given concomitant use of an epidural in study group and technical deviation from typical technique (only one intercostal level above and below underwent cryoneurolysis vs. the standard two levels).
Starting 2012, Clemence et al21 performed a retrospective cohort study on 117 patients undergoing open thoracic or thoracoabdominal aortic aneurysm repair. Twenty-five patients received cryoneurolysis. The cryoneurolysis group had reduced average narcotic usage by 28 MME in 10 days after extubation (p=0.005), but there were no differences in pain scores. Those receiving cryoneurolysis had more hours intubated (24 vs. 18 hours, p=0.3), longer intensive care unit stay (141 vs. 119 hours, p=0.4), and longer length of stay (15 vs. 11 days, p=0.03). Limitations included the retrospective nature, high risk of surgeon bias given selection of cryoneurolysis was based on surgeon preference, and differences in the nature of surgery and incision type between groups (higher percentage of urgent cases and thoracoabdominal incision in the cryoneurolysis group).
PICO 2 overall recommendation: In patients requiring thoracotomy for a thoracic procedure, IC compared with conventional pain modalities leads to decreased narcotic use (GRADE 2A) and pain scores (GRADE 2C) postoperatively. Impact of IC on hospital length of stay is not known.
Post-thoracotomy pain syndrome
PICO 3: in adult patients with PTPS, does image-guided percutaneous IC lead to improvement in symptoms?
For patients with PTPS, overall quality of evidence for IC was very low with three descriptive, single-centered studies (table 3). Two recent studies from 2010 (New York) and 2020 (Missouri) evaluated CT-guided IC and found improved pain scores after the procedure. One study from the UK in 1986 assessed ultrasound (US)-guided IC but performed no quantitative analysis.
Post-thoracotomy pain syndrome
In 1986, Conacher22 performed a descriptive study on 14 patients undergoing US-guided percutaneous cryotherapy for persistent post-thoracotomy neuralgia. Four consecutive intercostal nerve levels encompassing the area of discomfort were ablated. Three months after therapy, 50% of patients had improved symptoms, 21% had no change, and 29% had worsening pain. In those who got relief, symptoms were alleviated for a mean of 4.6 weeks (range: 2–12 weeks). Limitations include that intercostal cryoablation technique at this time was likely dated with obsolete technology given significant improvement in US equipment during the past four decades.
In 2010, Moore et al23 performed a descriptive study on 18 patients ranging from 36 to 78 years old with history of PTPS undergoing CT-guided cryoneurolysis. Time of insult ranged from 85 to 5113 days. Pain scores significantly improved from 7.5 preprocedure to 1.2 (p<0.0001) immediately postprocedure and 4.1 (p=0.005) at follow-up (range 5–157 days, mean 51 days). No postprocedure complications were noted. One patient reported recurrent pain within a week after significant relief at an intercostal level lower than procedure level, so the additional level was ablated with subsequent complete pain relief. Limitations included a descriptive study design without control and lack of data regarding number and level of intercostal levels ablated.
In 2020, Yasin et al24 presented a descriptive study of 13 patients aged 41–70 years undergoing CT-guided intercostal nerve cryoneurolysis for refractory PTPS. The intercostal level at the incision, two levels above, and two levels below were ablated. At 2 weeks postintervention, median pain scores decreased by three points (range −1 to 8, p=0.003). One patient developed pneumothorax requiring a chest tube and three reported pseudohernia, or subjective swelling, of the anterior abdomen. This study is limited by its descriptive nature without control, small sample size, and lack of long-term follow-up.
PICO 3 overall recommendation: For patients with PTPS, CT-guided percutaneous cryoneurolysis may lead to short-term pain relief but there are not enough data to make this recommendation.
Chest wall malignancy
PICO 4: in adults with chronic pain from malignancy involving the chest wall, does image-guided IC lead to reduction in pain?
Overall quality of evidence for IC in chest wall malignancy was very low with one single-center retrospective study in 2020 and one descriptive study in 2015, both from France (table 4). Both studies found improved pain scores after the procedure.
Malignancy
In 2015, Mavrovi et al25 performed a descriptive study of 32 cryoneurolysis procedures performed on 12 adult patients with refractory malignant neurologic chest pain. Median number of intercostal nerves undergoing cryoneurolysis was 3 (range 3–5).25 Mean VAS scores were significantly lower on postoperative days 1, 7 and 14. There were no major complications and two incidences of minor hematoma that resolved spontaneously. The major limitation was overall lack of detail in methodology, analysis, and results.
In 2020, Daubié et al26 conducted a retrospective review of 27 CT-guided cryoneurolysis procedures performed on 26 adult patients for refractory pain from malignancy. Mean preprocedural pain score was 6.5±1.7 and decreased to 2.4±2.4 on postoperative day 1, 1.8±1.7 on postoperative day 7 (p<0.001), 3.3±2.5 on postoperative day 14, and 3.4±2.6 on postoperative day 28 (p<0.05). Median pain relief was 45 days (range 14–70 days), and at the point of pain recurrence, 85% of patients had tumor progression. Two minor complications occurred—one patient experienced transient hypotension and the other experienced unexplained knee pain. There were no reports of neuritis or neuroma. Limitations included the retrospective nature of the study, small sample size, and heterogeneity of malignancies.
PICO 4 overall recommendation: For patients with refractory neuropathic pain from chest wall malignancy, CT-guided cryoneurolysis may provide short-term symptom relief but there are not enough data to make this recommendation.
Traumatic rib fractures
PICO 5: in adult patients with severe, traumatic rib fractures, does IC lead to better pain control?
Overall quality of evidence for cryoneurolysis in traumatic rib fractures was very low with one multi-institutional retrospective review and one descriptive study, both of which found improved pain scores after intervention (table 5).
Traumatic rib fractures
In 2019, Finneran et al27 performed a descriptive study on five adult patients who underwent US-guided percutaneous IC for rib fracture pain. Two received additional local anesthetic block. Four of four patients reported improved pain scores and one patient who was intubated to procedure was able to be extubated 12 hours after cryoneurolysis. No complications including neuropathic pain were reported on 3-month follow-up. Limitations of this study include its descriptive nature, very small sample size, and inability to distinguish the effects of local anesthetic block in 40% of the patients studied.
In 2019, Zhao et al28 also performed a retrospective review of 13 adult patients who underwent surgical stabilization of rib fractures (SSRF) and video-assisted thoracoscopy surgery-guided IC. Median number of ribs fractured was 7 (range 4–11), number of ribs plated was 4 (range 3–6), and number of cryoneurolysis levels was 6 (range 3–7). Postoperative pain scores were lower compared with preoperative scores (4.9 vs. 6.9, p=0.03). Mean length of stay after surgery was 5.9±2.7 days. By 21.3±6.2 weeks, all patients had regained at least partial sensation to the chest wall; none reported the hypoesthesia as bothersome. One patient (8%) developed severe, lifestyle-limiting hyperesthesia for up to 3 months but resolved at 6 months. Eight of 13 (62%) developed transient, non-lifestyle-limiting dysesthesias including tingling, burning, cramping, or sharp pain, which resolved by 6 months. Limitations include lack of narcotic usage data and inability to distinguish the effects of SSRF and cryoneurolysis given concomitant application.
PICO 5 overall recommendation: In trauma patients with severe rib fractures, stand-alone percutaneous IC or concomitant cryoablation during SSRF may lead to better pain control after trauma, but there are not enough data to make this recommendation.
Discussion
Cryoneurolysis has emerged as an effective modality for pain control, particularly for patients with refractory chest wall symptoms where conventional therapies have failed. The risk of neuroma or pseudohernia formation is low as the cryoneurolysis procedure, when performed appropriately, does not damage the perineurium nor epineurium29; other nearby vital structures like vessels and bone remain protected.30 One reported complication of cryoanalgesia is postoperative neuralgia, which has been reported in up to 20% to 30% of patients after IC.31 However, none of the reviewed studies demonstrated increased rates of neuralgia. Altogether, IC appears to remain a low-risk pain modality for chest wall pain.
Due to this favorable risk-to-benefit profile in management of chest wall pain, IC may be able to help fill the critical need for durable, long-term pain control in patients with severe rib fractures who have failed medical management. In addition to causing significant acute morbidity and mortality,1 rib fractures have been associated with chronic pain and prolonged disability.32–34 Conventional pain medications including narcotics can be problematic as adequate analgesia must be balanced with the risks of oversedation and respiratory depression. Moreover, neuraxial35 and regional anesthesia36 blocks are effective but technically demanding and relatively short lived. Cryoneurolysis of intercostal nerves after rib fractures addresses these shortcomings through a minimally invasive, often concomitant approach with proven, long-term pain control.
However, existing data supporting IC in trauma patients are scant, and there is a need for additional studies in this specific patient population. Two subpopulations in particular should be evaluated: (1) patients undergoing SSRF and (2) those unable to undergo SSRF. For the former subpopulation, when surgical fixation is indicated, a prospective trial comparing SSRF to SSRF plus cryoneurolysis may elucidate incremental benefits of IC. Standardized SSRF and cryoneurolysis technique across participating institutions would be imperative. To address the latter subpopulation, a prospective randomized controlled trial assessing efficacy of early, percutaneous IC versus standard of care in the elderly is currently under way.37
Our study has several limitations. First, most reviewed studies had low quality of evidence with only seven controlled trials and the rest mainly retrospective or descriptive in nature. Moreover, four of seven controlled trials were conducted in the 1970–1980s, a time period which lacked the current principles of pain management including multimodal therapy, making its application to the modern patient difficult. These studies were also lacking the level of detail in methodology and statistical analysis as with more modern studies. Second, most studies were limited by small sample size and may have been underpowered, particularly when evaluating for differences in operative time or complication rates. Another key limitation was lack of long-term follow-up, particularly given the long-term effects of IC.
Conclusions
IC may decrease inpatient narcotic use and shorten hospital length of stay. Complication rates are low without significantly increased rates of neuralgia or pneumothorax. IC does not definitively increase operative time. These findings are mainly derived from studies evaluating the pediatric population undergoing repair for pectus excavatum and adult patients requiring thoracotomy. Current evidence for IC in traumatic rib fractures is very low. However, given the favorable risk to benefit profile, further studies to better elucidate the analgesic efficacy of both percutaneous and thoracoscopic IC in the trauma patient with severe rib fractures are warranted.
Data availability statement
Data are available in a public, open access repository
References
Footnotes
Contributors PIC: main author, literature search, study design, data collection, data analysis, data interpretation. JGM: data collection, data analysis, data interpretation, article writing. AP: data collection, data analysis, article writing. JC: study design, data review, data interpretation, critical revision. NNK: data interpretation, critical revision. JDF: critical revision, article writing.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.