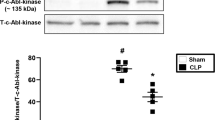
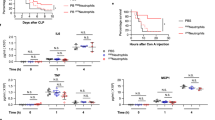
Abstract
Hyperinflammatory responses can lead to a variety of diseases, including sepsis1. We now report that extracellular histones released in response to inflammatory challenge contribute to endothelial dysfunction, organ failure and death during sepsis. They can be targeted pharmacologically by antibody to histone or by activated protein C (APC). Antibody to histone reduced the mortality of mice in lipopolysaccharide (LPS), tumor necrosis factor (TNF) or cecal ligation and puncture models of sepsis. Extracellular histones are cytotoxic toward endothelium in vitro and are lethal in mice. In vivo, histone administration resulted in neutrophil margination, vacuolated endothelium, intra-alveolar hemorrhage and macro- and microvascular thrombosis. We detected histone in the circulation of baboons challenged with Escherichia coli, and the increase in histone levels was accompanied by the onset of renal dysfunction. APC cleaves histones and reduces their cytotoxicity. Co-infusion of APC with E. coli in baboons or histones in mice prevented lethality. Blockade of protein C activation exacerbated sublethal LPS challenge into lethality, which was reversed by treatment with antibody to histone. We conclude that extracellular histones are potential molecular targets for therapeutics for sepsis and other inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wang, H. et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 (1999).
Bernard, G.R. et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344, 699–709 (2001).
Russell, J.A. Management of sepsis. N. Engl. J. Med. 355, 1699–1713 (2006).
Esmon, C.T. The protein C pathway. Chest 124, 26S–32S (2003).
Kerschen, E.J. et al. Endotoxemia and sepsis mortality reduction by non–anticoagulant-activated protein C. J. Exp. Med. 204, 2439–2448 (2007).
Mosnier, L.O., Zlokovic, B.V. & Griffin, J.H. The cytoprotective protein C pathway. Blood 109, 3161–3172 (2007).
Smirnov, M.D. & Esmon, C.T. Phosphatidylethanolamine incorporation into vesicles selectively enhances factor Va inactivation by activated protein C. J. Biol. Chem. 269, 816–819 (1994).
Riewald, M., Petrovan, R.J., Donner, A., Mueller, B.M. & Ruf, W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science 296, 1880–1882 (2002).
Taylor, F.B. et al. Protein-C prevents the coaggulopathic and lethal effects of Escherichia coli infusion in the baboon. J. Clin. Invest. 79, 918–925 (1987).
Zeerleder, S. et al. Elevated nucleosome levels in systemic inflammation and sepsis. Crit. Care Med. 31, 1947–1951 (2003).
Xu, J., Ji, Y., Zhang, X., Drake, M. & Esmon, C.T. Endogenous activated protein C signaling is critical to protection of mice from lipopolysaccaride-induced septic shock. J. Thromb. Haemost. 7, 851–856 (2009).
Lay, A.J., Donahue, D., Tsai, M.J. & Castellino, F.J. Acute inflammation is exacerbated in mice genetically predisposed to a severe protein C deficiency. Blood 109, 1984–1991 (2007).
Macias, W.L. & Nelson, D.R. Severe protein C deficiency predicts early death in severe sepsis. Crit. Care Med. 32, S223–S228 (2004).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
von Köckritz–Blickwede, M. et al. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 111, 3070–3080 (2008).
Clark, S.R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13, 463–469 (2007).
Beutler, B. & Cerami, A. The history, properties, and biological effects of cachectin. Biochemistry 27, 7575–7582 (1988).
Xu, J., Esmon, N.L. & Esmon, C.T. Reconstitution of the human endothelial cell protein C receptor with thrombomodulin in phosphatidylcholine vesicles enhances protein C activation. J. Biol. Chem. 274, 6704–6710 (1999).
Monestier, M., Fasy, T.M., Losman, M.J., Novick, K.E. & Muller, S. Structure and binding properties of monoclonal antibodies to core histones from autoimmune mice. Mol. Immunol. 30, 1069–1075 (1993).
Rittirsch, D., Huber–Lang, M.S., Flierl, M.A. & Ward, P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36 (2009).
Lupu, F. et al. Localization and production of plasminogen activator inhibitor-1 in human healthy and atherosclerotic arteries. Arterioscler. Thromb. 13, 1090–1100 (1993).
Acknowledgements
We thank K. Jackson for protein sequencing and identification; A. Matveev for preparing liposomes; C.J. Edgell, University of North Carolina, Chapel Hill, for providing EA.hy926 cells; G. Kinasewitz, University of Oklahoma Health Science Center, for providing plasma samples from humans with sepsis; K. Deatherage for preparing the manuscript; and P. Kincade for reviewing the original manuscript. C.T.E. is an investigator of the Howard Hughes Medical Institute. F.L. and F.B.T. are supported by US National Institutes of Health grant R01GM037704. C.T.A. and F.S. are supported by fellowships from the University of Bari, Italy.
Author information
Authors and Affiliations
Contributions
J.X. designed and executed many of the experiments; X.Z. participated in the experimental execution and contributed to experimental design; R.P. was involved in the initial experiments to activate the macrophages, leading to the release of histones; M.M. provided the antibodies to the histones and contributed useful comments; C.T.A. and F.S. performed the CLP experiments; F.B.T. provided the archival baboon sepsis plasma and contributed useful comments; N.L.E. made useful comments and assisted in manuscript preparation; F.L. provided constructive critiques of the studies and performed the calcium flux experiments and the histochemical analysis of the tissues; and C.T.E. oversaw the overall execution of the projects.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–4 and Supplementary Methods (PDF 402 kb)
Rights and permissions
About this article
Cite this article
Xu, J., Zhang, X., Pelayo, R. et al. Extracellular histones are major mediators of death in sepsis. Nat Med 15, 1318–1321 (2009). https://doi.org/10.1038/nm.2053
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2053
This article is cited by
-
Role and intervention of PAD4 in NETs in acute respiratory distress syndrome
Respiratory Research (2024)
-
Isoelectric trapping and discrimination of histones from plasma in a microfluidic device using dehydrated isoelectric gate
Microchimica Acta (2024)
-
Neutrophil extracellular traps and pulmonary fibrosis: an update
Journal of Inflammation (2023)
-
Neutrophil extracellular traps predict postoperative pulmonary complications in paediatric patients undergoing parental liver transplantation
BMC Gastroenterology (2023)
-
Targeting circulating high mobility group box-1 and histones by extracorporeal blood purification as an immunomodulation strategy against critical illnesses
Critical Care (2023)