Abstract
Objective
To compare intrathoracic blood volume (ITBV) guided fluid management and central venous pressure (CVP) guided therapy in ameliorating the progression of early systemic inflammatory response in patients undergoing major surgery.
Design
Prospective, randomized clinical trial.
Patients
Forty patients undergoing major abdominal surgery were randomized into CVP and ITBV groups.
Interventions
In the CVP group the target CVP was 8–12 mmHg while in the ITBV group the goal was to keep the ITBV between 850 and 950 ml/m2 during the operation.
Measurements and results
Hemodynamic parameters were determined by single arterial thermodilution. Measurements were repeated every 30 min intraoperatively. Serum procalcitonin (PCT) and C-reactive protein (CRP) was monitored preoperatively, on ICU admission, and then daily for 3 days. Serum TNF-α levels were measured intraoperatively hourly and then daily for 3 days. There was no significant difference between the two groups regarding hemodynamic parameters at any assessment point. In the overall population changes in the stroke volume index showed a significant correlation with changes in CVP and ITBV. TNF-α levels remained in the normal range intraoperatively and during the three postoperative days in both groups. Preoperatively normal PCT and CRP levels increased significantly postoperatively, without significant differences between the groups.
Conclusions
ITBV guided fluid therapy did not alter the magnitude of inflammatory response as monitored by serum PCT, CRP, and TNF-α in the early postoperative period.
Similar content being viewed by others
Introduction
Despite the advances in anesthesia and intensive care intraoperative hypovolemia may be one of the leading causes of postoperative morbidity following elective major abdominal surgery [1]. Inadequate intravascular volume status during surgery may lead to increased inflammatory response and further organ dysfunction due to gastrointestinal hypoperfusion [2, 3]. Several studies provide evidence that perioperative fluid optimization is beneficial for the patient [4, 5]. In addition to improving hemodynamics optimizing the patient’s intravascular fluid status may influence the immune response [6]. Therefore maintaining adequate cardiac filling throughout surgery seems an important task. However, controversy still exists concerning the best method regarding preload monitoring [7, 8, 9]. It has been shown that single transpulmonary thermodilution using the PiCCO method, which is less invasive than the pulmonary artery catheter, may provide adequate information about the patients’ volume status [10]. Della Rocca and coworkers [11, 12] suggest that the intrathoracic blood volume (ITBV) index (ITBVI) is superior to pulmonary artery occlusion pressure for indicating preload status during major thoracic and abdominal surgery. On the other hand, Rivers at el. [13] reduced mortality among septic patients with goal-directed therapy in which central venous pressure of 8–12 cmH2O was set as a hemodynamic goal to achieve SvO2 greater than 70%.
No clinical trial has yet investigated the effects of single transpulmonary thermodilution measurement guided fluid therapy on the intra- and postoperative inflammatory response and organ dysfunction. Therefore we conducted a clinical trial comparing standard central venous pressure (CVP) guided therapy to ITBV guided fluid management in patients undergoing elective major abdominal surgery.
Materials and methods
Clinical protocol
Following Regional Ethics Committee approval and obtaining written informed consent, all patients undergoing elective esophagectomy, total gastrectomy, pancreatectomy (Whipple operation), or liver resection due to tumor removal were entered the study and later admitted to our 20-bed teaching hospital high-dependency/intensive care unit between October 2002 and October 2003. Excluded were patients with chronic cardiovascular system failure (New York Heart Association class IV), ejection fraction less than 50% on resting transthoracic echocardiography, chronic respiratory failure (chronic hypoxia, hypercapnia), chronic renal failure requiring renal replacement therapy, chronic liver failure (biopsy-confirmed cirrhosis or portal hypertension), diabetes mellitus, or with known aortic aneurysm. Also withdrawn from the trial were patients whose tumor proved to be inoperable, who needed unexpected massive transfusion intraoperatively (>10 U blood) or required acute reoperation (within 24 h).
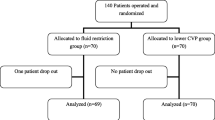
Forty-five patients were recruited during the study period and were randomly allocated by envelope randomization in a block-of-four fashion to CVP or ITBV. Due to our exclusion criteria five patients were withdrawn from the final analysis (three patients from the CVP group and two from the ITBV group, all due to tumor inoperability. Out of the remaining 40 patients there were 20 in each group (Fig. 1). The two groups were well matched for demographic and clinical characteristics on trial entry, and they did not differ significantly in terms of surgical procedures (Table 1).
In the CVP group the target CVP pressure was 8–12 mmHg during surgery [13]. In the ITBV group the desired end-point was to keep the ITBVI between 850 and 950 ml/m2 during the operation. In all patients lactated Ringer’s solution (10 ml/kg per hour) was infused as baseline volume replacement. Hydroxyethyl starch solution 6% (average molecular mass, 200 kDa; average molecular mass, 60,000; substitution ratio, 0.6; half-life, 24-h; Haes-Steril 6%, Fresenius-Kabi, Budapest, Hungary) or succinylated gelatin 4% (average molecular mass, 35 kDa; average molecular mass, 21,700; half-life, 4-h; Gelofusin, B.Braun Medical, Budapest, Hungary) was infused until the hemodynamic goals were achieved in both groups. In the CVP group the anesthetist responsible for the patient was unaware of the ITBV and the other hemodynamic parameters. In the ITBV group the anesthetist was aware of the ITBVI values, but blinded for CVP and the other hemodynamic variables. Patients were blinded for the result of randomization, as were the medical and nursing staff on the ICU.
All patients received routine anesthetic management and monitoring including: premedication with benzodiazepine, induction with propofol (1–2 mg/kg), muscle relaxation with atracurium besylate (0.4–0.6 mg/kg) analgesia with fentanyl (0.7–1 µg/kg). After induction of anesthesia an appropriately sized endotracheal tube was placed. Intermittent positive pressure ventilation was performed with a volumetric ventilator (ABT-4100, Kontron Instruments, Watford, UK). End-tidal CO2 and expiratory gases were monitored. Ventilation was adjusted to avoid gas trapping and dynamic hyperinflation with a tidal volume of 8–10 ml/kg and a respiratory rate of 10–12 breaths/min with a short inspiratory time and maximal expiratory time maintaining peak inflation pressures less than 40–45 cmH2O, with a fraction of inspired oxygen of 0.5. Positive end-expiratory pressure (PEEP) of 5 cmH2O was applied throughout. Anesthesia was maintained with isoflurane (0.7–1.5%), and continuous epidural analgesia (ropivacaine 2% combined with fentanyl) was used during surgery. Body temperature was controlled to avoid hypothermia with a warming blanket and warmed intravenous fluids.
Hemodynamic parameters were determined by single arterial thermodilution, for which a flexible catheter with an integrated thermistor (PiCCO, Pulsiocath 4F, PV 2024L, Pulsion Medical Systems, Munich, Germany) was introduced via the femoral artery after anesthesia was commenced. ITBV and extravascular lung water (EVLW) measurements were obtained by injecting a 20-ml bolus of 0.9% saline below 10°C via a central venous catheter. The mean value of three consecutive measurements was used for analysis. For interindividual comparison absolute values on ITBV and EVLW were normalized as indexed by body surface area (ITBVI, normal range 850–1000 ml/m2) and body weight (EVLW index, EVLWI, normal range 5–7 ml/kg). All injections were made manually and were not synchronized with the respiratory cycle. Cardiac index, stroke volume index (SVI), dP/dtmax, and systemic vascular resistance index (SVRI) were also recorded. Hemodynamic measurements were repeated in every 30 min. ΔCVP and ΔITBV values were calculated by subtracting the first from the second measurement, the second from the third and so on. Together with each hemodynamic measurement arterial blood samples were taken for blood gas analysis (ABL 700, Radiometer, Copenhagen, Denmark). Full blood count and blood loss were checked and recorded hourly during surgery.
Blood samples (approx. 5 ml) were taken from an indwelling arterial cannula into serum separator tubes before anesthesia was commenced (tp), hourly during surgery then on admission to ICU (tICU), and subsequently 24, 48, and 72h after admission (t24, t48, t72). Samples for measuring serum C-reactive protein (CRP), procalcitonin (PCT), and tumor necrosis factor (TNF) α levels were immediately spun after clot formation, and the serum separated and stored at −70°C. CRP levels were determined by nephelometric assay (Immage, Beckman, USA: reference range 0–10 mg/l). Serum PCT and TNF-α levels were measured by immunoluminometric assays (LUMItest, Brahms Diagnostica, Berlin, Germany, reference range 0–<0.5 ng/ml; or Immulite, DPC, USA, reference range 0–<10 pg/ml). The interassay coefficient of variation (CV) for both TNF-α and PCT determinations was less than 10%.
All patients had been observed after surgery for at least 2 days in the intensive care unit. Routine clinical and biochemical investigations were carried out. Patients were discharged from ICU if: SaO2 was 95% or higher on 35% O2 given via Venturi mask and good ability of cleaning bronchial secretions; multiple organ dysfunction score (MODS) was2 points or less; established enteral feeding via jejunostomy tube; and adequate analgesia. Each criterion had to be fulfilled for 12–24 h. To assess the treatment effect on organ function organ dysfunction scores were recorded on admission and then daily during the first 3 postoperative days (t0–t3) as described by Marshall et al. [14]. We designed our trial to assess effects of single transpulmonary thermodilution measurements guided fluid therapy on (a) the intra- and postoperative inflammatory response and (b) organ dysfunction.
Prior to the study the number of patients required in each group was calculated by power analysis according to data obtained from previous studies on the release of PCT in a similar population, in which the mean PCT was found to be 4.6±4.3 ng/ml 24 h postoperatively [2]. A previous descriptive study found that PCT values greater than 2.8 ng/ml showed an 84% sensitivity of predicting mortality [2]. Therefore the smallest difference between the means that we regarded as clinically acceptable not to be overlooked was 3.5 ng/ml (i.e., 4.6 for CVP and 1.1 ng/ml for ITBV). With type I α=5% and type II (power) of 80% we calculated we would need about 20 patients per group. All data are presented as mean ±SD or mean ±SEM. We used the Kolgomorov-Smirnov test with Lilliefor’s modification to test normal distribution. Analysis of variance was used for testing the significance levels between the different groups and analysis of variance for repeated measures was used for testing significance levels between the measurement stages. To investigate the relationship between the observed variables Spearman’s ρ was calculated. Data were analyzed comparing patients in the ITBV group with those in the CVP group on an intention-to-treat basis. For statistical analysis the Statistical Package for Social Sciences (SPSS version 10.0) software for Windows was used. Statistical significance was considered at p<0.05.
Results
The desired hemodynamic goals were reached in both groups. There was no significant difference between the CVP and ITBV groups regarding CVP, ITBVI, cardiac index, SVI, dP/dtmax, heart rate, or mean arterial pressure at any measurement point (Table 3). In the overall study population changes in the SVI showed a significant correlation with changes in CVP and ITBVI (r=0.288 p<0.001 and r=0.508 p<0.001, respectively (Fig. 2). There was no clinical evidence of fluid overload. EVLW was normal, and urine output was similar during the study period in both groups (Tables 2, 3). Patients in the two group received the same amount of crystalloid, colloid, and packed red blood cells (Table 2). Hemoglobin levels in neither group changed significantly during the study (Table 2). TNF-α levels remained in the normal range intraoperatively and increased slightly but not significantly during the three postoperative days in both groups (Fig. 3). Serum PCT levels were normal in both groups preoperatively and on arrival in the ICU (t0), but within 24 h levels increased significantly. Levels remained in the pathological range for the rest of the study with the maximum response observed at t24. There was no significant difference between the two groups (Fig. 4). Serum CRP levels were within the normal range preoperatively and on arrival on ICU in both groups but increased significantly in 24 h, with the maximum response observed at t48, without significant intergroup difference (Fig. 4). Regarding organ dysfunction the daily MODS did not differ significantly between the two groups throughout the study (Table 2). Median length of ICU stay, length of mechanical ventilation during the overall ICU stay, and survival were nearly identical in the two groups (Table 2).
Discussion
Intraoperative hypovolemia is common and may be a cause of organ dysfunction, increased postoperative morbidity and mortality [3, 15, 16, 17]. Goal-directed perioperative fluid management with the esophageal Doppler monitor (EDM) has been shown to reduce morbidity and length of hospital stay in patients undergoing open reduction of femur fracture and moderate-risk major surgery [4, 18, 19]. Moreover, EDM-guided fluid therapy augments gastric mucosal hypoperfusion, hence inflammatory response and complication rate following cardiac surgery [20]. Invasive hemodynamic monitoring with the PiCCO seems a reliable tool in assessing preload and is widely used in general intensive care [21]. However, no clinical trial has yet compared the effect of ITBV- vs. CVP-guided fluid management on postoperative inflammatory response in high-risk surgical patients. In our prospective randomized clinical trial we found no significant difference between the two groups regarding intra- or early postoperative inflammatory response and organ dysfunction. There are several factors that may influence our results.
Hemodynamics
It has been shown that early goal-directed therapy in which patients are volume-resuscitated to achieve CVP of 8–12 cmH2O significantly reduces mortality in patients at high risk for sepsis [13]. Therefore we chose to keep the CVP in this range in the control group while the manufacturer’s reference range was aimed at in the ITBV group. Preset hemodynamic goals were achieved in both groups. Interestingly, regarding the overall study population both ΔCVP and ΔITBVI showed significant correlation with ΔSVI. Several studies have compared CVP, ITBV, and pulmonary arterial occlusion pressure as indicators of preload [11, 12, 21, 22, 23]. Studies in favor of volumetric measurement of the cardiac filling have been performed mainly in septic, mechanically ventilated patients [21, 22]. In the current study CVP was almost identical to ITBV, which may be due to the different patient population. However, our data also challenge the very recent observation of Kumar and coworkers [23] in which CVP did not reflect cardiac filling and changes in SVI due to saline loading in healthy volunteers. Despite these differences between the current and previous investigations no firm conclusion can be made as this study was neither aimed nor designed and powered to answer the question whether ITBV or CVP is the better monitoring tool of cardiac filling during major abdominal surgery.
Inflammatory response
Previous investigations, including our own, have shown that major abdominal surgery which carries a considerable risk of morbidity and mortality initiates an inflammatory response as detected by increased level of various acute-phase proteins [2, 24, 25, 26]. PCT an infection-induced protein has been shown to be one of the most sensitive and specific early markers of sepsis [27]. Several authors have shown that the main stimulus of PCT production is the presence of bacterial endotoxin [27, 28]. All of our patients received prophylactic antibiotic treatment, and none showed clinical signs of infection over the study period. However, there is strong evidence that raised PCT levels can also be found after trauma, various type of surgery, and even cardiac arrest in the absence of microbiologically confirmed bacterial infection [2, 29, 30, 31]. It has been speculated that rise in PCT production can be induced if tissue hypoperfusion is present [29, 30, 31]. Furthermore, Meisner and coworkers [29] reported that the degree of hypovolemia during major surgery is correlated with elevated PCT levels in the postoperative period. Although there was no significant difference between the two groups, the postoperative PCT levels were lower in this study than in our recently published randomized controlled trial in a similar patient population and in published reference ranges [24, 26]. One of the possible explanations is that patients in the current study were volume resuscitated by target values, reducing the risk of hypoxic tissue damage, while this was not the task in the previous trials, and this itself attenuated the inflammatory response. In our previous investigation where N-acetylcysteine failed to attenuate the postoperative inflammatory response following major abdominal surgery, the CVP in the overall patient population on ICU admission was 8±4 mmHg vs. 13±5 mmHg in the current study (p=0.002). MAP and heart rate values which were also recorded did not differ significantly [24]. Interestingly, the total fluid volume administered (including blood products) in the previous trial was 4230±1270 ml vs. 5610±2954 ml in this study (p=0.043). Therefore it is likely that meticulous fluid replacement itself was responsible for the observed lower PCT levels.
This hypothesis is further supported by the findings that TNF-α levels were not elevated during surgery, and we observed only a minor and nonsignificant rise postoperatively in both groups. The main stimulus of TNF-α production is the presence of bacterial endotoxin [32]. In previous investigations TNF-α production was significantly lower in endotoxin-tolerant animals subjected to hemorrhagic shock than in normal subjects, suggesting little TNF-α activation in hypovolemia [33]. Furthermore, in a recent clinical trial normal levels were measured during elective abdominal surgery whereas a significant rise in TNF-α levels has been detected in patients with infection undergoing major surgery [34]. On the other hand, Buttenschoen et al. [25] reported transient endotoxemia and a transient reduced endotoxin inactivation capacity of the plasma associated with major abdominal surgery. It is possible that adequate fluid resuscitation achieved in both groups of our patients reduced the incidence of tissue, especially gut hypoperfusion, and hence inhibited the significant bacterial translocation and endotoxin challenge during the operation, as TNF-α production was not induced significantly during and after uncomplicated abdominal surgery.
CRP is a rapidly reacting (approx. 24 h) acute-phase protein synthesized by hepatocytes under the effect of bacterial infection, shock, and tissue damage [35, 36]. The predictive value of this inflammatory marker in critically ill patients is controversial [35, 37]. The extent of acute-phase response and CRP production is in proportion to the tissue damage during surgery [30, 38]. It has been shown that CRP levels are significantly lower in patients undergoing endovascular aortic aneurysm repair than in those undergoing the conventional, open procedure in which patients require significantly a greater amount of blood transfusion, indicating intraoperative hypovolemia [38]. In the present trial CRP levels increased significantly after surgery, and levels peaked on the second postoperative day without significant difference between the groups. This kinetic has been reported in several trials including our own [24, 26, 29]. However, the CRP levels measured in both our groups were lower than the previously published reference range and also compared to our recent study on the same patient population [24, 26]. Again, as the only difference between these trials was the vigorous attempt to avoid hypovolemia in the present study, we assume that the maintenance of adequate intravascular volume itself attenuated the inflammatory response in both groups.
Regarding outcome, the study population was too small to make any comments on length of stay, days of intermittent positive pressure ventilation, and mortality, and it was also not our aim to do so.
In conclusion, our results show that in patients at high risk of developing postoperative organ dysfunction following major abdominal surgery PiCCO-guided intraoperative fluid resuscitation compared to CVP-guided therapy fails to attenuate inflammatory response as monitored by serum PCT, CRP, and TNF-α in the early postoperative period.
References
Tandon S, Batchelor A, Bullock R, Gascoigne A, Griffin M, Hayes N, Hing J, Shaw I, Warnell I, Baudouin SV (2001) Peri-operative risk factors for acute lung injury after elective oesophagectomy. Br J Anaesth 86:633–638
Szakmany T, Molnar Z. Procalcitonin levels do not predict mortality following major abdominal surgery (2003) Can J Anaesth 50:1082–1083
Bennett-Guerrero E, Welsby I, Dunn TJ, Young LR, Wahl TA, Diers TL, Phillips-Bute BG, Newman MF, Mythen MG (1999) The use of a postoperative morbidity survey to evaluate patients with prolonged hospitalization after routine, moderate-risk, elective surgery. Anesth Analg 89:514–519
Sinclair S, James S, Singer M (1997) Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: randomised controlled trial. BMJ 315:909–912
Garrison RN, Wilson MA, Matheson PJ, Spain DA (1996) Preoperative saline loading improves outcome after elective, noncardiac surgical procedures. Am Surg 62:223–231
Boldt J, Muller M, Heesen M, Neumann K, Hempelmann GG (1996) Influence of different volume therapies and pentoxifylline infusion on circulating soluble adhesion molecules in critically ill patients. Crit Care Med 24:385–391
Wilson RJ, Woods I (2001) Cardiovascular optimization for high-risk surgery. Curr Opin Crit Care 7:195–199
De Backer D, Creteur J, Vincent JL (2003) Perioperative optimization and right heart catheterization: what technique in which patient? Crit Care 7:201–202
Bryson GL (2003) Best evidence in anesthetic practice. Goal-directed therapy with the pulmonary artery catheter is not better than standard therapy. Can J Anaesth 50:614–616
Della Rocca G, Costa MG, Coccia C, Pompei L, Di Marco P, Vilardi V, Pietropaoli P (2003) Cardiac output monitoring: aortic transpulmonary thermodilution and pulse contour analysis agree with standard thermodilution methods in patients undergoing lung transplantation. Can J Anaesth 50:707–711
Della Rocca G, Costa MG, Coccia C, Pompei L, Pietropaoli P (2002) Preload and haemodynamic assessment during liver transplantation: a comparison between the pulmonary artery catheter and transpulmonary indicator dilution techniques. Eur J Anaesthesiol 19:868–875
Della Rocca G, Costa GM, Coccia C, Pompei L, Di Marco P, Pietropaoli P (2002) Preload index: pulmonary artery occlusion pressure versus intrathoracic blood volume monitoring during lung transplantation. Anesth Analg 95:835–843
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M (2001) Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ (1995) Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 23:1638–1652
Mythen MG, Webb AR (1994) Intra-operative gut mucosal hypoperfusion is associated with increased post-operative complications and cost. Intensive Care Med 20:99–104
Shoemaker WC, Wo CC, Thangathurai D, Velmahos G, Belzberg H, Asensio JA, Demetriades D (1999) Hemodynamic patterns of survivors and nonsurvivors during high risk elective surgical operations. World J Surg 23:1264–1270
Anonymous (1999) Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology 90:896–905
Gan TJ, Soppitt A, Maroof M, el-Moalem H, Robertson KM, Moretti E, Dwane P, Glass PS (2002) Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 97:820–826
Price J, Sear J, Venn R (2002) Perioperative fluid volume optimization following proximal femoral fracture. Cochrane Database Syst Rev CD003004
Mythen MG, Webb AR (1995) Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg 130:423–429
Sakka SG, Ruhl CC, Pfeiffer UJ, Beale R, McLuckie A, Reinhart K, Meier-Hellmann A (2000) Assessment of cardiac preload and extravascular lung water by single transpulmonary thermodilution. Intensive Care Med 26:180–187
Lichtwarck-Aschoff M, Beale R, Pfeiffer UJ (1996) Central venous pressure, pulmonary artery occlusion pressure, intrathoracic blood volume, and right ventricular end-diastolic volume as indicators of cardiac preload. J Crit Care 11:180–188
Kumar A, Anel R, Bunnell E, Habet K, Zanotti S, Marshall S, Neumann A, Ali A, Cheang M, Kavinsky C, Parrillo JE (2004) Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med 32:691–699
Molnar Z, Szakmany T, Koszegi T (2003) Prophylactic N-acetylcysteine decreases serum CRP but not PCT levels and microalbuminuria following major abdominal surgery. A prospective, randomised, double-blinded, placebo-controlled clinical trial. Intensive Care Med 29:749–755
Buttenschoen K, Buttenschoen DC, Berger D, Vasilescu C, Schafheutle S, Goeltenboth B, Seidelmann M, Beger HG (2001) Endotoxemia and acute-phase proteins in major abdominal surgery. Am J Surg 181:36–43
Lindberg M, Hole A, Johnsen H, Asberg A, Rydning A, Myrvold HE, Bjerve KS (2002) Reference intervals for procalcitonin and C-reactive protein after major abdominal surgery. Scand J Clin Lab Invest 62:189–194
Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C (1993) High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 341:515–518
Dandona P, Nix D, Wilson MF (1994) Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab 79:1605–1608
Meisner M, Tschaikowsky K, Hutzler A, Schick C Schutler J (1998) Postoperative plasma concentrations of procalcitonin after different types of surgery. Intensive Care Med 24:680–684
Mimoz O, Benoist JF, Edouard AR, Assicot M, Bohuon C, Samii K (1998) Procalcitonin and C-reactive protein during the early posttraumatic systemic inflammatory response syndrome. Intensive Care Med 24:185–188
Fries M, Kunz D, Gressner AM, Rossaint R, Kuhlen R (2003) Procalcitonin serum levels after out-of-hospital cardiac arrest. Resuscitation 59:105–109
Tracey KJ, Lowry SF, Cerami A (1988) Cachectin: a hormone that triggers acute shock and chronic cachexia. J Infect Dis 157:413–420
Ackermann M, Reuter M, Flohe S, Bahrami S, Redl H, Schade FU (2001) Cytokine synthesis in the liver of endotoxin-tolerant and normal rats during hemorrhagic shock. J Endotoxin Res 7:105–112
Tang GJ, Kuo CD, Yen TC, Kuo HS, Chan KH, Yien HW, Lee TY (1996) Perioperative plasma concentrations of tumor necrosis factor-alpha and interleukin-6 in infected patients. Crit Care Med 1996 24:423–428
Okamura JM, Miyagi JM, Terada K, Hokama Y (1990) Potential clinical applications of C-reactive protein J Clin Lab Anal 4:231–235
Thompson D, Milford-Ward A, Whicher JT (1992) The value of acute phase protein measurements in clinical practice. Ann Clin Biochem 29:123–131
Pettila V, Pentti J, Pettila M, Takkunen O, Jousela I (2002) Predictive value of antithrombin III and serum C-reactive protein concentration in critically ill patients with suspected sepsis. Crit Care Med 30:271–275
Odegard A, Lundbom J, Myhre HO, Hatlinghus S, Bergh K, Waage A, Bjerve KS, Mollnes TE, Aadahl P, Lie TA, Videm V (2000) The inflammatory response following treatment of abdominal aortic aneurysms: a comparison between open surgery and endovascular repair. Eur J Vasc Endovasc Surg 19:536–544
Acknowledgements
The authors are indebted to Miss Ibolya Orosz for her technical assistance. The study was partially funded by NKFP 1A/0026 research grant, Ministry of Education, Hungary.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szakmany, T., Toth, I., Kovacs, Z. et al. Effects of volumetric vs. pressure-guided fluid therapy on postoperative inflammatory response: a prospective, randomized clinical trial. Intensive Care Med 31, 656–663 (2005). https://doi.org/10.1007/s00134-005-2606-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2606-4