Article Text
Abstract
Objectives The opioid crisis has forced an examination of opioid prescribing and usage patterns. Multimodal pain management and limited, procedure-specific prescribing guidelines have been proposed in general surgery but are less well studied in trauma, where multisystem injuries and multispecialty caregivers are the norm. We hypothesized that opioid requirements would differ by primary type of injury and by age, and we sought to identify factors affecting opioid prescribing at discharge (DC).
Methods Retrospective analysis of pain management at a level II trauma center for January–November 2018. Consecutive patients with exploratory laparotomy (LAP); 3 or more rib fractures (fxs) (RIB); or pelvic (PEL), femoral (FEM), or tibial (TIB) fxs were included, and assigned to cohorts based on the predominant injury. Patients who died or had head Abbreviated Injury Scale >2 and Glasgow Coma Scale <15 were excluded. All pain medications were recorded daily; doses were converted to oral morphine equivalents (OMEs). The primary outcomes of interest were OMEs administered over the final 72 hours of hospitalization (OME72) and prescribed at DC (OMEDC). Multimodal pain therapy defined as 3 or more drugs used. Categorical variables and continuous variables were analyzed with appropriate statistical analyses.
Results 208 patients were included: 17 LAP, 106 RIB, 31 PEL, 26 FEM, and 28 TIB. 74% were male and 8% were using opiates prior to admission. Injury cohorts varied by age but not Injury Severity Score (ISS) or length of stay (LOS). 64% of patients received multimodal pain therapy. There was an overall difference in OME72 between the five injury groups (p<0.0001) and OME72 was lower for RIB compared with all other cohorts. Compared with younger (age <65) patients, older (≥65 years) patients had similar ISS and LOS, but lower OME72 (45 vs 135*) and OMEDC. Median OME72 differed significantly between older and younger patients with PEL (p=0.02) and RIB (p=0.01) injuries. No relationship existed between OMEDC across injury groups, by sex or injury severity. Patients were discharged almost exclusively by trauma service advanced practice clinicians (APCs). There was no difference among APCs in number of pills or OMEs prescribed. 81% of patients received opioids at DC, of whom 69% were prescribed an opioid/acetaminophen combination drug; and only 13% were prescribed non-steroidal anti-inflammatory drugs, 19% acetaminophen, and 31% gabapentin.
Conclusions Opioid usage varied among patients with different injury types. Opioid DC prescribing appears rote and does not correlate with actual opioid usage during the 72 hours prior to DC. Paradoxically, OMEDC tends to be higher among females, patients with ISS <16, and those with rib fxs, despite a tendency toward lower OME72 usage among these groups. There was apparent underutilization of non-opioid agents. These findings highlight opportunities for improvement and further study.
Level of evidence IV.
- opioid
- acetaminophen
- pain management
- accidental injuries
Data availability statement
No data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known on this topic
It is known that the opioid epidemic is an ongoing issue that across the country.
Opioids are often prescribed in trauma surgery settings, and it has been identified that there is considerable room for clarification of opioid prescribing methods.
What this study adds
This study shows that there is underused potential for injury type to be a factor in defining hospital opioid prescribing regulations and for the increased use of multimodal pain management.
How this study might affect research, practice, or policy
This research may be useful for developing new hospital opioid prescribing protocols.
It is a testament to the many factors that may be considered when analyzing current prescribing methods and overall, can contribute to the changes needed to control this public health issue.
Background
In 2017, the US Department of Health and Human Services declared that a public health emergency existed regarding the opioid crisis.1 This was in response to a steady rise in opioid overdose deaths that had continued for at least 18 years.2 Liberal prescribing practices for opioid analgesics in the USA have been variably attributed to: (a) misrepresentation by pharmaceutical companies of a low risk of misuse or addiction3; (b) the Joint Commission’s Pain Management Standards in 2001, emphasizing pain as a ‘fifth vital sign’4; and (c) the inclusion of questions related to pain management in patient satisfaction surveys used by the Centers for Medicare and Medicaid Services to determine hospital reimbursement rates.4 However, the prescribing is ultimately the responsibility of the healthcare professional.
Opioid analgesics have been at the foundation of pain management in emergency settings across the USA5 A recent study from a level I trauma center found that patients treated for major orthopedic injuries as a result of high energy mechanisms all received opioids at the time of discharge (DC) and had a higher probability of abuse and dependency.6 In specialties such as general surgery, multimodal pain management and data-driven, procedure-specific opioid prescribing guidelines have been proposed to limit exposure to and dependence on opioids.7 8 In contrast, the multisystem nature of trauma and the involvement of multispecialty consultants impacts the ability to implement standardized guidelines. Eid et al9 have documented the variation within a single hospital acute care surgical service. Additionally, there has been little evidence to guide the transition from inpatient management to outpatient pain therapy.
The purpose of this quality improvement study was to review analgesic usage patterns among patients on a trauma service in a single center and to determine the relationship between inpatient opioid usage and DC prescriptions. We hypothesized that opioid requirements would differ by primary type of injury and by age, and we sought to identify factors affecting opioid prescribing at DC.
Methods
Scripps Memorial Hospital La Jolla is an American College of Surgeons (ACS)-verified level II trauma center in the San Diego County trauma system. This was a retrospective observational cohort study. After obtaining approval from the Institutional Review Board, a retrospective analysis of pain management was performed. The study population included adult patients 18 years of age or older who were admitted to the trauma center between January and November 2018 with major thoracic, abdominal, or orthopedic trauma. Patients with incomplete data, pregnancy, incarceration, death during hospitalization, or head Abbreviated Injury Scale (AIS) >2 combined with Glasgow Coma Scale <15 were excluded. A total of 214 patients were identified for this study population, of which 208 met inclusion criteria and 6 were excluded.
Patients were assigned to cohorts based on their predominant injuries with AIS >2, categorized as follows: major thoracic trauma including three or more rib fractures (RIB); abdominal trauma requiring laparotomy (LAP); pelvic fracture (PEL), femoral fracture (FEM), or tibial fracture (TIB). Patients with AIS >2 in a region outside the injury of interest, or multiple injuries with AIS >2, were excluded. Available retrospective patient medical data were collected, including daily analgesic, anxiolytic, and sedative medications and the doses administered. Opioid analgesic doses were converted to oral morphine equivalents (OMEs) using the following conversion factors (all in mg unless otherwise stated): oral codeine=0.15; oral tramadol=0.1; oral hydrocodone=1; oral oxycodone=1.5; oral methadone 1–20 mg/day=4, 21–40 mg/day=8, 41–60 mg/day=10, 61–80 mg/day=12; oral hydromorphone=4; parenteral morphine=3; parenteral hydromorphone=15; parenteral fentanyl=0.2 μg; transdermal fentanyl=2.4 μg/hour.10 Multimodal pain therapy was defined as the ordering of three or more analgesic medications. The primary outcomes of interest were OMEs administered over the final 72 hours of hospitalization (OME72) and prescribed at DC (OMEDC). Data are presented as means or medians, where appropriate. Categorical variables were analyzed using a two-proportion z-test, Fisher’s exact test and χ2 test and continuous variables with an unpaired t-test, Wilcoxon test, Kruskal-Wallis test, and analysis of variance . A p value <0.05 was considered significant.
Results
Two hundred eight patients were included. One hundred fifty-four (74%) were male. Females were older than males (65 vs 51 years, p<0.0001) and had shorter length of stay (LOS) (4.5 vs 7.3 days, p=0.004). Sixteen (8%) were using opioids prior to admission. One hundred thirty-four (64%) patients received multimodal pain therapy. The injury cohorts included: 17 LAP, 106 RIB, 31 PEL, 26 FEM, and 28 TIB. None of the RIB patients had surgical stabilization of rib fractures. Among the orthopedic injury patients, 73% had operative management; this included 89% of TIB, 69% of FEM, and 61% of PEL patients. Injury cohorts varied by age but not Injury Severity Score (ISS) or LOS (table 1).
Comparison of five injury cohorts
With the exception of PEL, all injury types had OME72 that differed from all of the other patients without that injury type: LAP versus all others, p=0.04; FEM versus all others, p=0.03; TIB versus all others, p<0.01; RIB versus all others, p<0.0001; PEL versus all others, p=0.47.
Older patients (aged ≥65 years) had similar ISS and LOS, but lower OME72 (45 vs 135, p<0.0001) and OMEDC, compared with younger patients (aged <65 years) (table 2).
Comparison of age cohorts: younger (<65 years) versus older (≥65 years), n=208
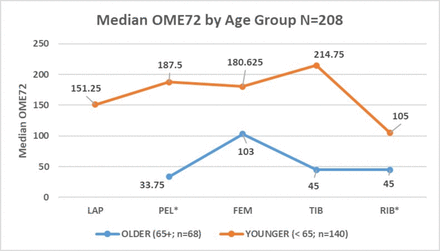
There was no difference between the cohorts in prescription of multimodal pain therapy (p=0.77). There was an overall difference in OME72 between the five injury groups (p<0.01) and OME72 was lower for RIB compared with all other cohorts, but OME72 did not vary significantly based on number of rib fractures. Median OME72 also varied by age within injury cohorts. The PEL (p=0.02) and RIB (p=0.01) differences were significant, but FEM (p=0.16) and TIB (p=0.48) were not (figure 1).
Median OME72 by injury cohort, comparing older (age >65 years) with younger (age <65 years) groups. *P<0.05, difference between age groups for injury cohort. FEM, femoral; LAP, laparotomy; OME72, oral morphine equivalents administered over the final 72 hours of hospitalization; PEL, pelvic; RIB, rib; TIB, tibial.
There was no relationship between OMEDC across injury groups, or by sex or injury severity. In fact, females and those with ISS <16 had slightly lower OME72 yet slightly higher OMEDC. In examining the effect of LOS, we found that the OME administered in the final 24 hours of hospitalization did not differ, nor did the number of pills prescribed at DC.
DC orders and prescriptions were written almost exclusively by trauma service advanced practice clinicians (APCs). There was no difference among the APCs in number of pills or OMEs prescribed. One hundred sixty-eight (81%) patients received opioids at DC; 69% of them were prescribed an opioid/acetaminophen (ACET) combination drug. Only 13% were prescribed non-steroidal anti-inflammatory drugs (NSAIDs) at DC, while 19% were prescribed ACET and 31% gabapentin (GABA).
Discussion
In this study, we found that opioid requirements varied by injury type, as well as between older adults and younger adults. We also found that opioid prescriptions at DC did not relate to the opioid usage in the final 72 hours prior to DC, indicating a disconnect in the transition from hospital to home. Finally, we found that the majority of patients received some form of multimodal pain therapy while inpatient, but only a minority were prescribed non-opioid agents at the time of DC. Thus, we have concluded that there are many opportunities to improve pain management and opioid usage after trauma.
Although our methodology differed, Harvin et al11 found similar variation by injury type from multiple centers. Our RIB cohort had the lowest OME72; in the Harvin study,11 the ‘flail chest’ cohort were second-lowest opioid users (after traumatic brain injury, which we excluded). We had much higher OME72 in TIB compared with FEM patients. Harvin et al11 combined long bone fractures, precluding direct comparison. Our LAP patients had the second highest OME72; LAP patients were also second highest in the study by Harvin et al.11 These findings raise the possibility that injury-specific factors should be studied further and potentially considered in pain management guidelines. In fields such as general surgery, protocols have been developed to establish procedure specific guidelines for opioid prescribing.7 8 Since the time that this study was performed, some multimodal pain guidelines have been established in trauma surgery.12–14 Indeed, one could argue that the recent prospective trial from Harvin et al13 demonstrates success of robust multimodal pain therapy across all types of patients, making it a moot issue. However, they tended to enroll multisystem injured patients in their trial so it might be worthwhile to prospectively study more isolated injury types. It is noteworthy that our patient cohorts did not differ in the total number receiving multimodal pain therapy. Unfortnately, we did not collect data on when multimodal pain therapy was initiated but this should be studied further.
We found that elderly patients received significantly fewer opioids than younger adults. This may have been a conscious decision on the part of the providers, but it is consistent with anecdotal observations that older patients often do not seem to complain of pain to the same degree. This was similarly reported by Hatton et al,15 in a secondary analysis of their prospective randomized trial. It is reassuring to note that similar pain control can be achieved with decreased opioid dosing.15 We had originally assumed the low OME72 in the RIB cohort was related to multimodal pain management. During the time period of our data collection, multimodal pain management had been promoted for the management of chest wall pain by both the Eastern Association for the Surgery of Trauma16 and the Western Trauma Association,17 and had been incorporated in our clinical care guideline for chest wall injury. However, it is possible that it could be related to the significant percentage (30%) of elderly patients in the RIB cohort. We will not be able to determine causality in this retrospective study, but the phenomenon is worthy of further study.
In this study, OME72 and OMEDC had no relationship across injury type, injury severity or gender, but did differ by age. In certain cases, there was even a negative correlation between OME72 and OMEDC. This indicates that prescribing patterns at DC are not taking any of our identified potential factors into account but are instead following rote methods. A recent review and meta-analysis by Zhang et al18 found that current guidance for the prescription of opioids at DC after abdominopelvic surgery is heterogeneous and rarely supported by evidence. Pelaez et al19 found that in a population of children, those with fractures required more opioids; they also noted that opioids were administered for a broad spectrum of injuries, including minor injuries. Bhashyam et al20 also found that fracture location was an independent predictor of the amount of opioids prescribed. Both cases exemplify a lack of systematic prescribing methods. Regardless of injury type or OME72, 81% of patients received opioids at DC, with 69% of them prescribed an opioid/ACET combination drug. The median OMEDC equated to 30 opioid/ACET combination pills. This was clearly an opportunity for improvement, as this practice potentially precludes the ability to take effective doses of ACET on a scheduled basis. Powelson et al21 reported that chronic pain after trauma may be predicted by postsurgical pain score at 6 hours, presence of a head injury, use of regional analgesia, and the number of postoperative non-opioid medications used for pain relief. We excluded patients with head injury and did not employ regional anesthesia. Further study is warranted to determine predictive factors beyond injury type and opioid requirements.
Current broad orthopedic guidelines dictate that any prescribing of long-term opioids should be limited to one prescriber. Furthermore, prescribing the lowest effective immediate release opioid for the shortest possible period is recommended. Regional anesthesia, psychosocial interventions and aromatherapies have all been suggested in the efforts to reduce opioid distribution.22 Warner et al23 assessed opioid prescribing practices after spine surgery and noted that despite recent efforts, there remain a few specific areas that warrant improvement. Foremost appears to be the prescribers’ ‘understanding of the role of opioid guidelines’. Each healthcare professional’s interpretation of opioid guidelines affects their personal prescribing habits. This is congruent with a study by Chapman et al,24 which notes that individual’s prescribing habits relate directly to the intensity of opioids received postoperatively. Other areas for improvement, according to Warner et al,23 include the transition of opioid prescribing responsibility between surgical and primary care teams, managing analgesic expectation of the patient (especially in patients with chronic pain), and opioid tapering. Each of these areas currently have wide ranges of inconsistency and no clear processes. Our study attempts to find a potential root to the inconsistencies and propose a way to begin forming tangible guidelines.
One area of focus to further reduce opioid prescribing is education and training in proper use and the implementation of protocol. In our evaluation of which healthcare professionals were prescribing most frequently, we discovered that the opioid prescriptions were being written nearly exclusively by our APCs and that there was no difference between APCs in their prescriptions. While this has not been well-studied in trauma, there appears to be significant variation in prescribing practices between physicians and APCs. Some studies have suggested overprescribing behavior among APCs, and particularly among emergency department patients with injury.25 26 This requires further study and intensive education of providers. Attention must be paid to surgical trainees as well. Data from a study affiliated with the Department of Orthopedic Surgery at Harvard found that surgical residents did most opioid prescribing, and that they often prescribe over the state law maximum.27 These are both examples of how opioid prescriptions are widely routine and lack personalization. Bhashyam et al27 go on to state that less than half of those prescribing opioids participated in an opioid training program. Other studies show that surgical residents either feel ‘inadequately trained’28 or feel that additional training on proper prescribing methods of analgesics would be beneficial.29 There are many factors that lead to higher levels of opioid prescribing, but with more education on pain management along with a standardized protocol, opioid use has the potential to be significantly reduced.30
Another area of focus to pair with proper protocols is emphasizing multimodal pain management. Of our patients, only 13% were prescribed NSAIDs, 19% ACET, and 31% GABA at DC. It is important to acknowledge that over-the-counter analgesics may not have been ‘prescribed’ but rather recommended at the time of DC. We did not collect complete data on such recommendations and thus may have underestimated the number who were given such a recommendation. However, the practice of the APPs at the time was to routinely prescribe an ACET/opioid combination drug, and thus additional ACET was not likely recommended. The use of some alternative analgesics such as GABA, ACET, and ibuprofen were significantly lower during hospital stay, and although prescribed at DC, were underused. Oyler et al14 state that education emphasizing the effectiveness of non-opioid pain management reduced opioid use at DC. This alludes to the importance of a protocol and one centered around non-opioid options.
This slow adoption of multimodal pain management in trauma surgery may have been multifactorial. In our institution, there was a pervasive concern about the safety of NSAIDs with regard to fracture healing. While some studies have suggested a potential concern, the evidence is of low quality and current opinion is that it should not subvert multimodal pain therapy.31 32 Sim et al33 show in postoperative outpatients after general surgery, lower amounts of opioids were necessary when combined with courses of ibuprofen or ACET. Additionally, Hamrick et al12 notes that when multimodal pain management is used properly, opioid use can be reduced without compromising any patient comfort. Gessner et al34 reviewed several multimodal pain management strategies including NSAID use. This literature recognizes that opioids may not be the most effective, although very cost effective, for ‘poly-trauma’. They instead claim that multimodal pain management can provide a higher standard of comfort so long as the prescriber is well versed in pain management and the care is personalized to each patient.
Limitations
This study has a number of limitations. It is retrospective and we did not record pain scores. We did not perform a power analysis and are likely underpowered to show differences between injury cohorts or age groups due to small numbers. We were unable to record all of the DC medications; as some non-opioid agents are available over the counter, it is possible that medications were recommended but not specifically prescribed. We also have no information regarding the number of OMEs that were used after DC.
The choice of OME72 as our primary outcome was somewhat subjective. We considered various alternatives to presenting the data, including OME for the entire hospital stay; average per day; and OME on the final day of hospital stay. We felt that a 3-day average would be the best of the options for a number of reasons. Compared with total OME, it would avoid major differences based on LOS variability. Compared with overall average per day, looking at the last 72 hours of hospitalization de-emphasized the immediate postoperative dosing, which might include more intravenous opioids and less oral non-opioid medications; we also felt it would better reflect the ‘steady-state’ amount that we were giving as the patient approached DC, allowing us to get a handle on the transition from inpatient to outpatient prescription. Compared with just looking at the final day, we wanted to avoid variation due to time-of-day DC (eg, a patient discharged at 10:00 hours vs 18:00 hours would likely have different OME given).
Conclusions
Patients with different injury types appear to have varied opioid requirements. Opioid DC prescribing does not correlate with actual opioid usage during the 72 hours prior to DC. Paradoxically, OMEDC tends to be higher among females, patients with ISS <16, and those with rib fractures, despite a tendency toward lower OME72 usage among these groups. ACET, NSAIDs and GABA are underused. These findings highlight opportunities for improvement and further study.
In recent years, federal and state legislations have stepped in to propose strategies of bringing some control to opioid access. This ultimately places the prescriber in control of limiting the amount of opioids prescribed for a given injury. The Food and Drug Administration has been forthcoming with their stance on opioid control and have stated that the epidemic will continue to grow unless there is a drastic change in clinician prescribing patterns.
Supplemental material
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Scripps Institutional Review Board (IRB-18-7283).
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Presented at AAST poster presentation.
Contributors The corresponding author (WLB) is the guarantor of this work. All authors have made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.