Article Text
Abstract
Background Non-compressible truncal hemorrhage (NCTH) is the leading cause of preventable death after trauma. Resuscitative endovascular balloon occlusion of the aorta (REBOA) achieves temporary hemorrhage control, supporting cardiac and cerebral perfusion prior to definitive hemostasis. Aortic zone selection algorithms vary among institutions. We evaluated the efficacy of an algorithm for REBOA use.
Methods A multicenter prospective, observational study conducted at six level 1 trauma centers over 12 months. Inclusion criteria were age >15 years with evidence of infradiaphragmatic NCTH needing emergent hemorrhage control within 60 min of ED arrival. An algorithm characterized by the results of focused assessment with sonography in trauma and pelvic X-ray was assessed post hoc for efficacy in a cohort of patients receiving REBOA.
Results Of the 8166 patients screened, 78 patients had a REBOA placed. 21 patients were excluded, leaving 57 patients for analysis. The algorithm ensures REBOA deployment proximal to hemorrhage source to control bleeding in 98.2% of cases and accurately predicts the optimal REBOA zone in 78.9% of cases. If the algorithm was violated, bleeding was optimally controlled in only 43.8% (p=0.01). Three (75.0%) of the patients that received an inappropriate zone 1 REBOA died, two from multiple organ failure (MOF). All three patients that died with an inappropriate zone 3 REBOA died from exsanguination.
Discussion This algorithm ensures proximal hemorrhage control and accurately predicts the primary source of hemorrhage. We propose a new algorithm that will be more inclusive. A zone 3 REBOA should not be performed when a zone 1 is indicated by the algorithm as 100% of these patients exsanguinated. MOF, perhaps from visceral ischemia in patients with an inappropriate zone 1 REBOA, may have been prevented with zone 3 placement or limited zone 1 occlusion time.
Level of evidence Level III.
- hemorrhage
- control
- algorithm
- aorta
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Severe hemorrhage remains the leading cause of preventable mortality in trauma patients.1–5 Non-compressible truncal hemorrhage (NCTH) is associated with an exceptionally high mortality rate; up to 85% in the military setting and approaching 50% in civilian patients.6 7 Patients with NCTH and subsequent hemorrhagic shock require rapid hemodynamic intervention to control bleeding and replace blood volume, while maintaining appropriate perfusion of vital organs in order to prevent exsanguination, terminal dysrhythmia and death. Research shows that resuscitative endovascular balloon occlusion of the aorta (REBOA) is feasible and effective as a hemorrhage temporizing maneuver to rapidly increase cardiopulmonary and cerebral perfusion, slow bleeding and serve as a bridge to definitive hemorrhage control in the operating room or interventional radiology suite.8–11
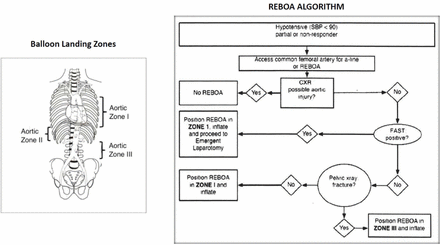
The current clinical algorithm for REBOA utilization in the USA calls for selective placement of the balloon in either zone 1 (thoracic aorta) for patients with presumed intra-abdominal or retroperitoneal hemorrhage or zone 3 (infrarenal aorta) in patients with presumed hemorrhage arising from the pelvis (see figure 1).12 13 Currently in Japan, where REBOA is an established form of intervention for patients with NCTH, the standard practice is to place the REBOA catheter in zone 1 despite the location of suspected hemorrhage.14 Aortic zone selection strategies vary among institutions, without consensus on the most effective algorithm for use. This study aims to optimize early decision-making regarding aortic zone selectivity in patients receiving REBOA. The objective of this study is to evaluate the efficacy of the aortic zone selection algorithm, depicted in figure 1, post hoc using ER-REBOA catheter (Prytime Medical, Boerne, Texas, USA) prospective data collected at major US trauma centers.
Aortic zones of deployment and current resuscitative endovascular balloon occlusion of the aorta (REBOA) algorithm. CXR, chest X-ray; FAST, focused assessment with sonography in trauma; SBP, systolic blood pressure.
Methods
Study design
This study evaluates the efficacy of a popularly used REBOA algorithm with regard to proximal hemorrhage control and accuracy of optimal zone prediction, post hoc, in a cohort of patients receiving REBOA from an original prospective, observational study. The algorithm is characterized by the results of focused assessment with sonography in trauma (FAST) and pelvic X-ray. Patients were excluded if there was no FAST exam, an indeterminate FAST exam, a positive cardiac FAST or unknown primary bleeding source. Outcomes were then assessed as to whether the algorithm was followed.
The original prospective, observational study was conducted at six US level 1 trauma centers from 31 May 2017 to 15 June 2018. Inclusion criteria of the original study included: (1) age 15 years or older; (2) evidence of truncal hemorrhage arising below the diaphragm in which the decision for emergent truncal hemorrhage control intervention (operative or endovascular) was made within 60 min of emergency department (ED) arrival and (3) presentation to one of the participating level 1 trauma centers at highest activation level. Prisoners were excluded.
Although data were collected on all patients meeting eligibility criteria and included varying hemorrhage control interventions, this descriptive post hoc data analysis includes only patients receiving REBOA. The zones of aortic occlusion are defined as follows: zone 1 is from the branch of the left subclavian artery to the celiac artery, zone 2 is from the celiac artery to the renal arteries and zone 3 is from the renal arteries to the aortic bifurcation. Zones 1 and 3 are the preferred zones of occlusion, while zone 2 is considered to be a no occlusion zone. Proximal hemorrhage control was defined in this study as REBOA deployment between the heart and primary hemorrhage source. All data elements of the original study were collected prospectively using direct observation.
Of the 8166 patients screened for enrollment during the original study period, 78 patients had a zone 1 or zone 3 REBOA placed. We acknowledge the possibility of selection bias in that the patients presenting to each of our six level 1 trauma centers involved in the study may be more severely injured and face greater mortality risk than the average trauma patient presenting at other institutions. In order to reduce the effect of this possible bias on our study, we have chosen to exclude patients that meet certain criteria that may represent those with potentially unsalvageable injuries. In addition, we have chosen to exclude patients, based on per-protocol analysis, which cannot be appropriately assessed with regard to the algorithm in question. Twenty-one of these patients were excluded for having any of the following characteristics: no FAST exam (five patients), an indeterminate FAST exam (seven patients), a positive cardiac FAST (three patients) or unknown primary bleeding source (nine patients). Although the FAST exam is typically performed with a cardiac view as a single component of the entire exam, we thought it pertinent to specifically identify patients that had a positive FAST in the cardiac view due to inapplicability of the algorithm being assessed in the setting of hemorrhage above the diaphragm. The patients with an unknown primary bleeding source includes patients died prior to surgery and patients the attending surgeon indicated had an unknown primary bleeding source during/after surgery. Primary bleeding source was an explicit data point provided by the attending surgeons of the original study that we used in our post hoc analysis. The remaining 57 patients are the subject of the following post hoc analysis.
Data management and statistical analysis
Study data from the original study were collected and managed using REDCap 23 and analyzed using JMP software, V.15 (SAS Institute, Cary, North Carolina, USA). Medians and IQR as well as proportions were calculated to summarize REBOA utilization, patient characteristics and outcomes. First, we separated the final post hoc study population into zone 1 and zone 3 REBOA groups. Then, we further subdivided those groups based on whether or not the algorithm was followed. Comparisons were made within each zone of REBOA deployment based on whether the algorithm was followed or violated (ie, zone 1, algorithm followed vs zone 1, algorithm violated). The subgroup characteristics and outcomes were tested at the p<0.05 level using the Wilcoxon rank-sum test for all continuous data and the Fisher’s exact test (0<n<5) and χ2 test (5<n<∞) for dichotomous data.
Results
The algorithm ensures REBOA deployment proximal to primary hemorrhage source to control bleeding in 98.2% of cases and accurately predicts the optimal REBOA zone in 78.9% of cases. If the algorithm was violated, the primary bleeding source was proximally controlled in only 43.8%, significantly less than the 98.2% rate with algorithm adherence (p=0.01). The remaining 1.8% of cases that the algorithm did not predict proximal hemorrhage control for were due to the rate of incorrect FAST, meaning false negative or false positive. The characteristics of each subgroup of patients receiving REBOA are depicted in table 1.
Patient characteristics of study comparison groups
Of the 36 patients treated with a zone 1 REBOA, 4 patients should have received a zone 3 REBOA, according to the algorithm. Zone 1 placement was able to be confirmed via imaging in 61.1% of patients, with balloon migration occurring in 13.6% of image-confirmed patients. The four patients that received an inappropriate zone 1 REBOA had a median time to complete aortic occlusion of 65 min and a median time to anatomic hemostasis of 4 hours, 23 min. The remaining 32 patients that received an appropriate zone 1 REBOA had a median time to complete aortic occlusion of 22 min (IQR=14–46 min) and a median time to anatomic hemostasis of 2 hours, 6 min (IQR=74–209 min). Three of the patients that incorrectly received a zone 1 died (75.0%). The most common primary cause of mortality in these patients was multiple organ failure (MOF) with a frequency of 66.7% among those that died with a median time to death of 159 hours. Twenty of the 32 patients that had an appropriately placed zone 1 REBOA, according to the algorithm, died with a mortality rate of 62.5% (p=0.62). The most common primary cause of mortality in these patients was exsanguination, with a 75.0% frequency among those that died with a median time to death of 1 hour. There is no statistically significant difference in mortality in patients with an inappropriately placed zone 1 REBOA (p=0.62).
Of the 21 remaining patients treated with a zone 3 REBOA, only 8 (38.0%) were appropriate as per the algorithm. The remaining 13 patients (62.0%) should have received a zone 1 REBOA according to the algorithm. The 13 patients that received an inappropriate zone 3 REBOA had a median time to complete aortic occlusion of 32 min (IQR=19–35 min) and a median time to anatomic hemostasis of 1 hour, 51 min (IQR=66–193 min). The remaining eight patients that received an appropriate zone 3 REBOA had a median time to complete aortic occlusion of 22 min (IQR=17–29 min) and a median time to anatomic hemostasis of 1 hour, 50 min (IQR=86–260 min). Mortality was 23.1% in patients with an inappropriate zone 3 REBOA and 25.0% in those with an appropriately placed zone 3 REBOA (p=0.92) (table 2). None of the patients with an appropriately applied zone 3 REBOA died within 24 hours of admission. All of the zone 3 mortalities in which a zone 1 was indicated, died within 24 hours of admission. Of those three patients with an inappropriate zone 3 REBOA that died, two had a positive FAST exam and all died from exsanguination with a median time to death of 6 hours (table 3). One of the FAST positive, zone 3 patients that exsanguinated had penetrating trauma to the pelvis with primary source of hemorrhage being the left common iliac vein and only minor mesenteric arterial injury. Two patients with an appropriate zone 3 REBOA died, one from TBI and the other due to unknown causes with a median time to death of 249 hours.
Mortality rates compared between appropriate and inappropriate zone deployment, according to the algorithm
Rates of exsanguination among patients that died compared between appropriate and inappropriate zone 3 deployment, according to the algorithm
With regard to concerns about potential consequences of unnecessary organ ischemia in a zone 1 REBOA with a primary source of bleeding in the pelvis, we compared the overall incidence of acute kidney injury (AKI) and MOF in all zone 1 and zone 3 patients receiving REBOA, excluding only those that died within the first 24 hours following ED admission. Thirty-nine per cent of patients with a zone 1 REBOA (n=23) suffered AKI, compared with 40.0% of patients with a zone 3 REBOA (n=25; p=0.95). Furthermore, MOF occurred in 26.1% of zone 1 patients and 4.0% of zone 3 patients (p=0.03). Additionally, when considering only the zone 1 patients that did not die within the first 24 hours after ED admission, the total occlusion time between balloon catheter inflation and complete deflation varied between those that did develop MOF and those that did not. Patients that did not develop MOF (n=15) had a median occlusion time of 41 min (IQR=24–71). Patients that developed MOF (n=6) had a median occlusion time of 100 min (IQR=69–109.3).
Discussion
The principal goal of REBOA is to provide proximal control of severe NCTH. The algorithm assessed in this study, if followed 100% of the time, would ensure increased incidence of REBOA deployment proximal to primary hemorrhage source, while also reducing the risk of unnecessary visceral organ ischemia by accurately predicting the optimal zone of deployment. The optimal REBOA zone is defined as the zone being immediately proximal to the primary source of hemorrhage. For example, if the primary source of hemorrhage were in the pelvis, a zone 3 would be considered both proximal and optimal, while a zone 1 would be considered proximal, but not optimal. This study did not exhibit statistically significant differences between patients in which the algorithm was followed versus those in which the algorithm was violated, within each zone. It is worth noting that the small sample size within each category of compared patients could have contributed to the statistically insignificant results.
Patients that received a zone 3 REBOA (n=21) had a mortality rate of 23.8% while patients that received a zone 1 REBOA (n=37) had a mortality rate of 62.2%. The difference in mortality between zone 3 and zone 1 patients is most likely a result of pre-existing disparities due to the initial traumatic injury, although complications associated with each selected zone may have some role. Median systolic blood pressure (SBP) on ED admission was 75 mm Hg in zone 3 patients receiving REBOA and 60 mm Hg in zone 1 patients receiving REBOA and median serum lactate on ED admission was measured to be 7.25 and 9.1, respectively. It is also worth noting that 69.6% of zone 1 mortalities were due to exsanguination, none of which would likely have been prevented with a zone 3. The inherent differences between patients that received a zone 1 and those that received a zone 3 warranted the need for the two groups to be analyzed separately, with regard to algorithm adherence/violation.
The decision regarding the initial REBOA zone of occlusion is made based on readily available information including the patient’s blood pressure (SBP <90 mm Hg), presence of intra-abdominal fluid on FAST exam and plain X-ray of the pelvis (demonstrating presence of pelvic fracture). Potential benefits of selective occlusion include avoidance of visceral hypoperfusion for patients with isolated pelvic hemorrhage treated with a zone 1 REBOA. However, inflation of the balloon in zone 3 in patients with intra-abdominal or retroperitoneal hemorrhage could theoretically worsen hemorrhage by inflating the balloon below the level of hemorrhage, thereby increasing aortic arterial pressure leading to worsened blood loss. Based on this principle, we recommend that even in the setting of an apparently isolated penetrating pelvic injury, yet a positive FAST exam, zone 1 REBOA should be performed instead of zone 3. In this study, the only zone 3 patient that exhibited penetrating pelvic injury with a positive FAST, despite only minor mesenteric injury, died from exsanguination. Additionally, there is a high false negative rate of the FAST exam and the inability for FAST to evaluate for hemorrhage in the retroperitoneum.15
Reflecting the general lack of consensus within the trauma community regarding algorithm usage, providers in this study occasionally violated the algorithm. Although no statistical difference in outcomes was detected, accuracy of placement would certainly have been greater had all providers adhered to the algorithm, which we suspect could influence outcomes in large-scale study. We find the data to warrant revisitation of the currently established algorithm, depicted in figure 1, to facilitate greater buy-in from REBOA users. With regard to the patient population that was able to be analyzed in this study, the large number of patients receiving REBOA that required exclusion in order to analyze the algorithm may elicit concern regarding the limited inclusivity of the algorithm. The only subcategory of excluded patients for which we can provide potentially meaningful suggestions regarding algorithm modifications would be for patients with an indeterminate FAST exam. In these patients, we would suggest performing a diagnostic peritoneal aspirate (DPA) and substituting the results for the results of the FAST exam, or performing a zone 1 REBOA straightaway. One additional correction that we would make to the algorithm would be to include a directive for patients that exhibit a (+) cardiac FAST exam, in which REBOA would be contraindicated. Based on these considerations, we propose a new algorithm for REBOA deployment strategy that is depicted in figure 2, for both blunt and penetrating trauma.
Proposed resuscitative endovascular balloon occlusion of the aorta (REBOA) algorithm for blunt and penetrating trauma. CXR, chest X-ray; FAST, focused assessment with sonography in trauma; SBP, systolic blood pressure.
There is well-warranted concern regarding the potential contribution of prolonged zone 1 REBOA to the development of AKI and/or MOF, especially in a patient that could receive proximal hemorrhage control with a zone 3. As detailed above, patients receiving zone 1 REBOA are typically sicker and would likely have high rates of AKI and MOF even in the absence of REBOA. The current rates of AKI and MOF in patients with hemorrhagic shock approach 43% and 30%, respectively.16 17 The rate of AKI in REBOA, specifically, has been previously reported to be 19% in a cohort with an in-hospital mortality rate of 63%.18 Although the rate of AKI in the subjects of our study is much higher, the total mortality rate is much lower (49.1%). We find it paramount to note that complications such as AKI and MOF can only develop in a patient that is alive and in the ICU.
Although we find it unlikely that zone 1 REBOA, with appropriate occlusion time, contributes directly to the development of AKI and MOF, we maintain that zone 3 deployment has a place in the arsenal of REBOA strategies and should continue to be used for patients with an isolated pelvic bleed. Additionally, based on the association of MOF with longer zone 1 occlusion times, attempts at hemorrhage control should be performed in a timely manner to reduce total occlusion time. However, we also find it important to emphasize the principal goal of REBOA—to temporize bleeding, allowing us to get definitive hemorrhage control in the operating room and then deal with the potential consequences of organ ischemia and shock in the ICU. Performing a zone 3 REBOA on a patient with a (+) FAST exam, in the interest of reducing the risk of MOF, neglects the principal aim of REBOA.
In order to better prevent and/or treat MOF in these patients, we believe the next best step would be to study the problem of MOF more specifically, without jeopardizing our ability to prevent exsanguination. There are many possible contributing factors in the development of MOF in these patients that we believe can and should be teased apart with further large-scale study. Here are a few topics we think would be worth exploring in studies with a larger sample size: the role of periodic partial balloon deflation, the confounding variable of severe hemorrhagic shock, the potential role of reperfusion injury versus ischemia and subsequent therapeutic development potential.
This study has several limitations. First, a sizeable portion (n=21) of an already limited number of patients (n=78) had to be excluded from the analysis of the algorithm to appropriately limit selection bias and adhere to per-protocol analysis. This is the most significant limitation of the study and we highly recommend further, large-scale study. Second, there is no control group for comparison. Third, the results may not be generalizable being that the majority of patients in the study were admitted to high-volume trauma centers.
This observational study was funded by the US Department of Defense to better characterize the range of traumatically injured patients in whom the ER-REBOA catheter could be used and to gain a better understanding of the early decision making and procedural details related to utilization and safety of this technology. This study was not designed to compare the effectiveness of various temporary or definitive hemorrhage control procedures or the outcomes of patients undergoing hemorrhage control interventions.
Conclusion
The evaluated algorithm, based on FAST exam and pelvic X-ray data, ensures proximal hemorrhage control and accurately predicts the primary source of hemorrhage. We propose a new algorithm that will be more inclusive of patients with an indeterminate FAST exam. Patients with an indeterminate FAST exam should receive a DPA to act as a substitute for FAST exam data in the algorithm, or a zone 1 REBOA straightaway. A zone 3 REBOA should not be performed when a zone 1 is indicated by the algorithm as 100% of the mortalities were due to exsanguination, which may have been prevented with zone 1 placement. The incidence of MOF, perhaps from visceral ischemia in patients with an inappropriate zone 1 REBOA, may have been prevented by limiting total zone 1 occlusion time or zone 3 placement.
Acknowledgments
The authors would like to thank the collaborators for their contributions to this study.
References
Footnotes
Collaborators Emergent Truncal Hemorrhage Control Study Group: Coordinating Center: Laura J. Moore, MD; Charles E. Wade, PhD; Erin E. Fox, PhD; Jeanette M. Podbielski, RN; Xun Xu, MS; Stacia M. DeSantis, PhD; Jada Johnson, MS. Clinical sites (listed in order of number of patients enrolled): University of Washington: Eileen M. Bulger, MD; Patricia Klotz, RN; Nick Opgenorth. University of Texas Health Science Center at Houston: David Meyer, MD; Ezra Koh; Laura Vincent, RN. Shock, Trauma and Anesthesiology Research-Organized Research Center (STAR-ORC), RAdams Cowley Shock Trauma Center, University of Maryland Medical Center: Thomas M. Scalea, MD; Philip Wasicek. Emory University: Bryan Morse, MD; LaShondra DeYampert. University of Southern California: Kenji Inaba, MD; Monica D. Wong, MS; Yvonne Hojberg. Denver Health: Charles Fox, MD; Alexis Cralley; Joshua Ryon; Konrad Ben; Nick Brant.
Contributors All authors listed have contributed significantly to this work.
Funding The Emergent Truncal Hemorrhage Control Study was sponsored by Prytime Medical Devices, Inc., through a contract with the US Department of Defense (W911QY-15-C0099).
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval This study was approved by the US Institutional Review Board (HSC-GEN-17-0055), with waiver of informed consent.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon request.