Article Text
Statistics from Altmetric.com
A 64-year-old woman who had been hit by a train presented to the emergency department with bilateral upper limb and left lower limb crush injuries. The patient’s pulse was 134 beats per minute; blood pressure, 78/44 mm Hg; and respiratory rate, 30 breaths per minute. She was intubated, and initial resuscitation was started immediately.
During the primary survey, a left-sided hemothorax causing hemorrhagic shock was noted and massive bloody discharge via a chest drain was observed. She underwent left thoracotomy and wedge resection of the injured lung using a staple device in the left lower lobe. In addition, she underwent bilateral forearm and left above knee amputation. The gas exchange deteriorated intraoperatively owing to massive transfusion and lung contusion. Veno-venous extracorporeal membrane oxygenation (VV-ECMO) was initiated without heparin. An extracorporeal circuit was constructed in a veno-venous configuration, with femoro-jugular flow direction (figure 1). Consequently, the hemodynamic status and gas exchange improved rapidly. On transfusing 10 L of crystalloids and 50 units of blood products, the VV-ECMO blood flow gradually reduced to 0.8 L/min and blood oxygen saturation deteriorated to 68%. Based on the patient’s overall condition, the massive bleeding had likely been arrested. The abdomen was distended, but focused assessment with sonography for trauma was negative for hemoperitoneum and ascites.
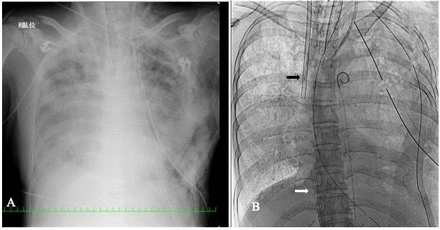
Chest roentgenogram with veno-venous extracorporeal membrane oxygenation (VV-ECMO) cannulas. (A) Chest roentgenogram shows bilateral opacification due to lung contusion and multiple rib fractures. (B) ECMO cannulas were placed using fluoroscopy. The black arrow marks the cannula placed at the right atrium via the right jugular vein, and the white arrow marks the cannula placed at the inferior vena cava via the right femoral vein.
What would you do?
A. Reposition the VV-ECMO drainage cannula.
B. Transfuse more units of blood products.
C. Change the ventilator setting.
D. Measure the intra-abdominal pressure.
What we did and why?
D. Measure the intra-abdominal pressure.
Thoracoabdominal X-ray showed no cannula malposition. Intra-abdominal pressure (IAP) measured via the bladder was 85 mm Hg. Ultrasound evaluation revealed collapse of the inferior vena cava (IVC) around the cannula. We diagnosed secondary abdominal compartment syndrome (ACS), which could have worsened the ECMO blood flow. She was emergently transferred to the operating room (OR) for decompressive laparotomy with vacuum pack closure. There was no blood, but massive bowel edema and minimal serous fluid were noted in the abdomen (online supplementary digital content 1). Subsequently, the IAP decreased and VV-ECMO blood flow and gas exchange improved (table 1). Ultrasound revealed sufficient IVC space around the cannula. After 48 hours of ECMO, lung compliance and oxygenation recovered. She was weaned from ECMO and underwent tracheostomy. She returned to the OR 2 days later and underwent definitive abdominal closure. She was ultimately discharged to a skilled nursing facility.
Supplemental material
The parameter changes before and after decompressive laparotomy
VV-ECMO is gradually becoming common for traumatic respiratory failure, but the practical way to use ECMO remains unknown in trauma patients. The most common cause of reduced ECMO flow in trauma patients is intravascular volume depletion caused by severe bleeding. It is not widely known that ACS can cause reduced ECMO flow. Some reports state that intra-abdominal hypertension (IAH) can complicate ECMO management. Reduced ECMO flow may cause physicians to make an incorrect assessment of the fluid status if ACS is not considered. Excessive fluid loading should be avoided as it exacerbates gas exchange. As large volume resuscitation is one of the major risk factors for IAH development, IAP should be measured when ECMO flow decreases in severe trauma patients. In this case, massive transfusion could have caused pulmonary deterioration and IAH development. Minimization of crystalloid fluid resuscitation with balanced blood product resuscitation might have prevented ACS and gas exchange deterioration.
Cannula malposition and line obstruction are other causes of reduced ECMO flow. Besides IAP measurement, transabdominal ultrasound of the IVC can help assess the cause of reduced ECMO flow. Using ultrasound, we assessed the IVC diameter around the ECMO femoral drainage cannula. Additionally, we confirmed appropriate positioning and the absence of issues, including a thrombus.
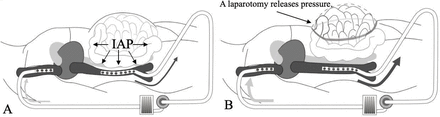
The pathophysiology of IAP affecting ECMO flow is as follows. In a typical configuration of the VV-ECMO circuit, the blood is drained from the IVC via femoral cannulation under negative pressure and transferred to the pump and oxygenator, after which it is returned to the right atrium. Increased IAP is related to the IVC diameter. Therefore, blood drainage can be impaired owing to IAH (figure 2A). In this case, decompressive laparotomy was effective for improving ECMO flow, which released the IVC compression associated with massive abdominal edema (figure 2B).
Effect of intra-abdominal pressure on veno-venous extracorporeal membrane oxygenation (ECMO) flow. (A) Massive bowel edema compresses the inferior vena cava and reduces blood flow to the heart (preload). As a result, drainage blood flow for ECMO is impaired. (B) Decompressive laparotomy releases intra-abdominal hypertension and improves ECMO flow. IAP, intra-abdominal pressure.
The other common type of ECMO is venoarterial ECMO (VA-ECMO). VA-ECMO is suitable for cardiogenic shock or arrest. In the setting of trauma, the most common cause of shock is hypovolemia due to hemorrhage. Therefore, there are limited chances for VA-ECMO use in trauma patients. In either a VA or VV-ECMO case, blood drainage can be impaired owing to IAH and decompressive procedures are sometimes required. Therefore, a switch to VA-ECMO could not overcome the issue in our case.
In summary, we demonstrated troubleshooting of the ECMO circuit in a trauma patient. ACS can cause decreased ECMO flow in trauma patients.
Footnotes
Contributors Article drafting: SM. Critical revision: MM, MY, MK. Figure preparation: KM.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.