Article Text
Abstract
Background Complementopathy (rapid complement activation and consumption after trauma) has been reported in trauma patients, but the underlying mechanism of these phenomena and their clinical significance remain unclear. This study aimed to determine the complement/complement pathway activation and identify the association of complement activation with clinical outcomes in trauma patients.
Methods We studied 33 trauma patients with mean Injury Severity Score of 25, and 25 healthy volunteers. Sera were collected on patients’ arrival at the emergency department, as well as 1, 2, 3, 5, and 7 days after trauma, to measure the levels of terminal complement activation product soluble C5b-9 (sC5b-9) by ELISA. In addition, the functional complement activation pathway was evaluated using a commercial complement system screening kit.
Results Serum concentrations of sC5b-9 (complement terminal pathway activity) were significantly increased in trauma patients throughout the entire observation period except on day 1. Complement terminal activities were significantly higher in 27 of 33 patients with systemic inflammatory response syndrome (SIRS) than non-SIRS patients on day 2, day 5, and day 7. Increased serum levels of sC5b-9 positively correlated with SIRS. Functional complement analysis revealed that the classical pathway was the predominant pathway responsible for complement activation. Burn patients tended to have a greater and prolonged classical pathway activation than non-burn patients, and burn injury and blunt injury were associated with higher blood levels of sC5b-9 than penetrating injury.
Discussion Early complement activation through the classical pathway after trauma is observed and positively correlated with the development of SIRS. Thus, monitoring of the complement system might be beneficial in the care of critically injured patients.
Level of evidence III.
Study type Prognostic.
- complement system
- trauma
- prognosis
- systemic inflammatory response syndrome
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Background
The complement system, as a central component of innate immunity, helps phagocytic cells to kill and clear invading pathogens. Although first discovered as an effector arm of the antibody response, complement can also be activated early in infection in the absence of antibodies. The complement system consists of more than 30 proteins in the serum and on cell surfaces. Complement activation results in the generation of C3a, C3b, and C5a, and in the formation of C5b-9 (a lytic complex that inserts into the membrane, forming a pore in the cell). In addition to cell lysis, the cascade also increases phagocytosis via C3b, recruits inflammatory cells through the anaphylatoxins C3a and C5a, and instructs the adaptive immune system to produce an appropriate humoral response.1 2 Physiologic complement activation represents an extremely effective means of pathogen lysis, thereby preventing infection as well as participating in both inflammatory initiation and resolution. However, inappropriate or exaggerated complement activation and rapid consumption may act as a double-edged sword, resulting in excessive tissue destruction and organ damage while destroying pathogens. Such dysfunction of the complement cascade (ComC) termed as “complementopathy” frequently occurs in patients with traumatic injury.1 3 4
After trauma, the complement system, coagulation system, neutrophils, and the cytokine/chemokine network are triggered and orchestrate the “first line of defense” of innate immunity, thus initiating a systemic danger response to overcome the insult. The complement system is considered to be a major driver of systemic and local inflammation, and has been implicated in pathologic conditions or complications typically seen in severely injured patients, including systemic inflammatory response syndrome (SIRS), sepsis, acute respiratory distress syndrome, multiple organ dysfunction syndrome (MODS), and ischemia-reperfusion injury.1 2 5–7 Trauma patients may die as a consequence of their initial injuries or of the subsequent MODS associated with their immune-inflammatory responses.8 However, the mechanism by which trauma induces complement activation and its clinical significance remain obscure.
In general, three cascade pathways (the classical complement pathway, the alternative complement pathway, and the mannose-binding lectin [MBL] pathway) activate the complement system.9 The common end product of this complement activation cascade is the generation of a multimer terminal complement complex C5b-9 (also known as membrane-attack complex), which is made of C5b, C6, C7, C8, and C9. The terminal complement complex C5b-9 mediates irreversible cell membrane damage and/or cellular activation. Complement complexes formed in the absence of a target membrane bind to a naturally occurring regulatory serum protein, the S protein (vitronectin). The S protein binds to nascent C5b-9 complexes at the C5b-7 stage of assembly, resulting in soluble C5b-9 (sC5b-9), a stable, non-lytic form of the complex, which can be detectable with ELISA.10 The measurement of sC5b-9 by ELISA is usually used as a measure of the intensity of complement activation in diseases. However, it remains unknown whether sC5b-9 levels could predict clinical outcomes of trauma patients. The present study assessed whether any such relationship exists between sC5b-9 levels with occurrence and severity of pathologic conditions after trauma, such as SIRS, sepsis, or MODS, in trauma patients.
Methods
This was a single-center, prospective, cohort study of major trauma patients presenting directly to level 1 trauma center.
Patients and healthy subjects
Thirty-three patients with mean Injury Severity Score (ISS) of 25 were studied as part of a prospective cohort study of trauma patients admitted to Brooke Army Medical Center. Twenty-five healthy volunteers over 18 years of age were recruited as the control subject cohort. The following were the inclusion criteria for healthy volunteers: (1) free of acute illness for at least two previous weeks; (2) minimal 50 Kg weight; and (3) no pregnancy. Patients (>18 years old) who were admitted to the intensive care unit (ICU) within 24 hours of injury and expected to stay in the ICU at least 72 hours were enrolled in the study. For the enrollment of patients, the following exclusion criteria were applied: (1) admission period greater than 24 hours from time of injury; (2) admission to the hospital ward; (3) prisoners; (4) pre-existing therapeutic anticoagulation (exception made for aspirin or ibuprofen); and (5) coagulopathy, or other underlying condition prior to trauma. Volunteers donated a one-time blood sample after signing a consent form in accordance with regulatory policy.
Clinical data
Clinical and laboratory data were collected during the first 7 days after patients’ admission into the emergency department (ED).11 Injury mechanism (blunt, penetrating, or burn), percent burned, inhalation injury, ventilator-free/ICU-free/hospital-free days, fluid administration, time of injury and arrival in the ED, and mortality were entered into an Oracle Database. Laboratory results and other basic demographic data were recorded. Daily MODS, defined as a Marshall score of 10 or greater, was determined based on clinical and laboratory parameters.12 SIRS was evaluated and defined based on adult SIRS criteria according to clinical manifestations including body temperature, heart rate, tachypnea, arterial partial pressure of carbon dioxide, white cell count, hyperglycemia, and altered mental state. When two or more of these criteria are met with or without evidence of infection, patients were diagnosed with SIRS.13
Blood collection
Blood samples were collected into serum tubes via central venous or arterial catheters within 24 hours of admission into the ED and on days 1, 2, 3, 5, and 7 after injury. Blood sampling was discontinued on transfer out of the ICU or the removal of central venous and arterial catheters. The blood samples were centrifuged, and sera were collected with standard procedure, aliquoted and stored in cryoprecipitate polypropylene tubes in −80°C freezers for subsequent analysis. Blood samples collected from healthy volunteers were processed in the same manner.
Analysis of complement and pathway activation
Blood levels of complement activation product C5b-9 (sC5b-9) were determined by ELISA (MicroVue SC5b-9 Plus EIA Kit, Quidel, San Diego, CA). Functional complement classical, lectin, and alternative pathways were examined using the Wieslab complement system screening kit (Euro Diagnostica, Malmo, Sweden). The complement activation pathway identifying assay combines the hemolytic assay for complement activation with antibodies specific for the neoantigen of the complement complex produced during activation. The wells of the assay plates were coated with activators specific to the classical, the MBL, or the alternative pathway. These specific activators initiated the corresponding activation pathways in serum samples, finally resulting in the formation of the terminal complement complex C5b-9. The amount of C5b-9 product is proportional to the functional activity of complement pathways. The amount of complement activation correlated with the color intensity in the assay wells and was measured in terms of absorbance as values of optical density (OD). Functional activity for the respective pathways was then calculated using the OD values of the positive control (PC) and negative control (NC) provided in the kit as follows: (sample − NC)/(PC − NC)×100. A lower percentage reflected a higher complement activation/consumption.
Statistical analysis
Group data were expressed as means±SEM. Serum complement levels were compared using one-way analysis of variance followed by Tukey’s multiple comparison test or unpaired t-test with Welch’s correction. P values <0.05 were considered significant for all analyses.
Results
The analysis included 33 trauma patients and 25 healthy volunteers. The clinical characteristics and demographics of the trauma patients and healthy volunteers are shown in table 1.
Clinical and demographics data
Increase in C5b-9 levels and complement activation after trauma
Serum levels of sC5b-9 were measured to assess terminal complement activation. The results showed significantly higher sC5b-9 concentration in sera at admission compared with the healthy volunteers (figure 1). Dramatic elevations of sC5b-9 occurred by day 2 and remained more than twofold higher than the levels at the time of admission through day 7. Such increase of sC5b-9 level indicates a robust activation of the complement system and a complement consumption burst in the first week after trauma. Of note, burn and blunt injuries resulted in higher blood levels of sC5b-9 than penetrating injury (data not shown).
Serum concentrations of sC5b-9 in patients after trauma. Serum sC5b-9 levels were measured by ELISA assays. Data are expressed as mean±SEM. *p<0.05, ***p<0.001 versus healthy volunteers (n=25). n=33, n=24, and n=19 for ED, D5, and D7, respectively. D1, day 1; D2, day 2; D3, day 3; D5, day 5; D7, day D7; ED, emergency department; sC5b-9, soluble C5b-9.
The classic pathway is the predominant pathway for complement activation after trauma
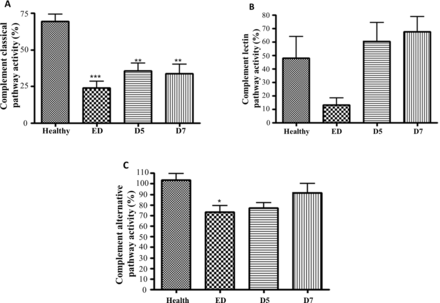
Results of the Wieslab complement system screening assay showed that the classic pathway is the one that predominates over the complement activation in injured patients, as evidenced by the remarkably lower percentage activity levels (24% at admission, p<0.001; 36% on day 5, p<0.01; 34% on day 7, p<0.01) in their serum samples compared with the healthy subjects (69%) (figure 2A). In contrast, no significant differences were observed in the percentage activities of the other two pathways in the majority of time points, except in the case of admission in the alternative pathway assay (figure 2B,C). In the assay for the alternative pathway, we observed a significant difference in trauma patients as compared with healthy subjects only at the time point of admission (p<0.05), but not on day 5 and day 7, indicating that this pathway may be activated only in the very early stage after trauma (figure 2C). Furthermore, we also found that patients with burns had greater classical pathway activation (D5-D7) and higher lectin pathway activities at the ED than non-burn patients (data not shown).
Functional complement pathway activation in trauma patients. The complement pathway activation was measured using the Wieslab complement system screen kit: (A) classic pathway; (B) lectin pathway; and (C) alternative pathway. Data are shown as the mean OD ratio of serum samples to a positive control of each group. A lower OD value reflects higher intensity of corresponding pathway activation that has happened before blood sampling. Data are expressed as mean±SEM. *P<0.05, **p<0.01, ***p<0.001 versus healthy volunteers (n=25). n=33, n=24, and n=19 for ED, D5, and D7, respectively. D5, day 5; D7, day 7; ED, emergency department; OD, optical density.
Relationship of C5b-9 with SIRS in trauma
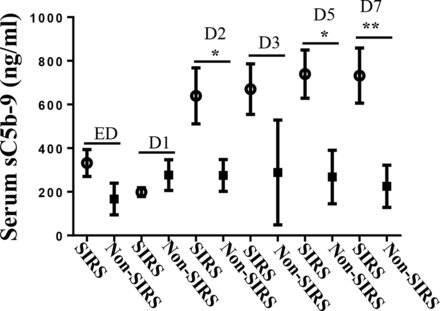
In the current study 27 of 33 trauma patients developed SIRS (94%) (table 1). sC5b-9 levels at ED tended higher in patients with SIRS than in patients without SIRS. Higher levels of serum sC5b-9 were associated with SIRS, reaching statistical significance on day 2 (639.4±128.4 versus 275.2±72.8, p<0.05), day 5 (739.3±110.4 versus 268.3±122.4, p<0.05), and day 7 (732.2±126.3 versus 225.4±96.6, p<0.01) (figure 3).
Comparisons of sC5b-9 serum concentration in trauma patients without (n=6) or with (n=27) SIRS. Data are expressed as mean±SEM. *P<0.05, **p<0.01. D1, day 1; D2, day 2; D3, day 3; D5, day 5; D7, day 7; ED, emergency department; sC5b-9, soluble C5b-9; SIRS, systemic inflammatory response syndrome.
Serum levels of sC5b-9 and functional complement pathway activation in different types of injured patients
In this study, 12 of 33, 15 of 33, and 6 of 33 patients were diagnosed with burn injury (36%), blunt injury (46%) and penetrating injury (18%), respectively (table 1). Burn injury and blunt injury were associated with higher blood levels of sC5b-9 than penetrating injury (figure 4A), and burn patients tended to have a greater and prolonged classical pathway activation than non-burn patients, but differences did not achieve statistical significance due to small sample size.
Comparisons of serum sC5b-9 concentration and functional complement pathway activation in different types of trauma patients. Serum sC5b-9 concentration in thermal trauma (A, n=12), blunt trauma (B, n=15), and penetrating trauma (C, n=6) patients. Functional complement pathway activation in thermal trauma (D, n=12), blunt trauma (E, n=15), and penetrating trauma (F, n=6) patients. Data are expressed as mean±SEM. *P<0.05, **p<0.01, ***p<0.001 versus healthy; †p<0.05 versus ED. AP, alternative pathway; CL, classical pathway; D1, day 1, D2, day 2; D3, day 3; D5, day 5; D7, day 7; ED, emergency department; LP, lectin pathway; sC5b-9, soluble C5b-9.
No relationship between sC5b-9 levels and ISS, MODS, hypotensive resuscitation (data not shown), hospital stay, ICU stay, or ventilation was observed (data not shown).
Discussion
The data from this study relating complement status in trauma patients revealed that (1) sC5b-9 levels were significantly increased throughout the entire 7-day observation period except on day 1; (2) the classical pathway of complement activation predominates during the first week after trauma; and (3) serum sC5b-9 levels are positively correlated with SIRS.
Trauma remains a leading cause of death worldwide. Hemorrhagic shock and direct tissue damage after trauma activate a multifaceted network of plasma cascades including ComC. The early ComC activation and consumption after severe trauma play critical roles in the derangement of immune system that is characterized by a biphasic pattern: early SIRS and subsequently systemic anti-inflammatory response syndrome (SARS). SIRS and SARS precipitate injury-related early and late MODS, respectively.14 Several studies addressed the detrimental facets of post-trauma complement activation and tried therapy targeting complement inhibition.15–17 Indeed, excessive complement activation leads to the development of early MODS via triggering inflammation, vascular leak, cell lysis, thrombosis, and acidosis. Exhaustion of the complement system may contribute to the development of late MODS due to immunosuppression and infection. Extensive complement activation after trauma also leads to elevated plasma levels of C3a, C5b-9, and C5a. Increased levels of C3a, C5b-9, and C5a correlated directly with injury severity, MODS, and mortality.18 Furthermore, C5a induces neutrophil activation resulting in autologous endothelial and mesothelial damage.19 In addition, in sepsis excessive C5a production caused cellular and organ dysfunction on multiple levels, eventually leading to a poor outcome.20 21 However, the underlying mechanism of these phenomena and their clinical significance remain unclear. Therefore, further studies to determine the timeframe of complement activation/depletion and complement pathway activation after trauma are definitely needed for diagnosing complementopathy based on the specific status of individual patient. This will provide precise guide for complement modulation (supplementary or inhibition) therapy.
The first important finding of our study is the emergence of classical pathway as the predominant activated complement pathway, which was unexpected. A plausible explanation is that specific binding of natural IgM antibodies to post-trauma expressed neoantigens triggers activation of complement classical pathway. Several studies have demonstrated that the binding of natural IgM antibodies with apoptotic and necrotic cells leads to the activation of the classic pathway.22 23 Indeed, a body of documented evidence exhibits that specific self-reactive IgM antibody initiates activation of classic pathway, associated with tissue damage in the animal models of burn trauma and ischemia-reperfusion injury.24–26 Data of the present study support the notion that the classic pathway is mainly associated with complement activation observed in trauma except in the very early stage. Our investigation demonstrates that an alternative pathway may play an important role in the very early stage after trauma (figure 2C). In previous studies the alternative pathway was reported to be a central pathway for the amplification of the complement activation within 30 minutes after trauma, whereas classical or lectin pathway played a role in triggering initiation of the activation.27 In the pathway screening assay, our data still showed a possibility that the lectin pathway was involved in the earlier phase of trauma-induced complement activation (figure 2B), whereas the classic pathway was working along the entire observing period of 7 days after patient admission. However, this needs to be confirmed in a larger patient cohort.
The second important finding of our study is that sC5b-9 levels are positively correlated with SIRS. Trauma often induces systemic and local inflammation. As the “first line of defense,” the complement system is a crucial entity of innate immunity and a major initiator of inflammatory response. Post-traumatic ischemia-reperfusion injuries activate ComC that plays an important role in sustaining the SIRS via its chemoattracting leukocytes, and crosstalking with coagulation/kinin/fibrinolytic cascades and innate/adaptive immunity. Excessive complement activation after major trauma triggers SIRS that leads to MODS and death. Recently, multiple preclinical and clinical studies have demonstrated that complement activation after major trauma plays critical role in contributing to the SIRS and MODS in traumatic brain injury, chest trauma, musculoskeletal trauma, and polytrauma.28–31 Taken together, the complement activation products represent the main drivers of the systemic inflammation after trauma. However, we did not find a significant correlation between sC5b-9 and MODS or hypotensive resuscitation in this small patient cohort. This needs to be confirmed in a larger patient cohort.
In this study, we also found that burn injury and blunt injury were associated with higher blood levels of sC5b-9 than penetrating injury, indicating that profound release of damage-associated molecular patterns and/or pathogen-associated molecular patterns after burn and blunt injuries may contribute to the different immune response. Burn patients tended to have a greater and prolonged classical pathway activation than non-burn patients, suggesting that consumption of components of the classical pathway after burn injury may at least partially contribute to the overall classical pathway activation in this cohort study.
Limitations of this study
Since this was a single-center and small observational cohort study of major trauma patients, a much larger patient cohort should be enrolled to determine the correlations of individual complications of trauma. In addition, we have monitored patients 7 days after trauma, and the changes in complement activation may persist longer or occur later. Also, interaction and crosstalk between complement and damage-associated molecular patterns, pathogen-associated molecular patterns, coagulation system, and specifically natural IgM antibody need to be investigated. In the future, large, multiple-center, prospective, and randomized studies are necessary to further address these pressing issues.
In conclusion, we demonstrated that complement activation occurs early after trauma, and the classical pathway is the predominant pathway of such activation. The levels of sC5b-9 are associated with SIRS and aging. sC5b-9 levels may be a feasible and useful prognostic biomarker for complications after trauma. Monitoring the chronologic changes of sC5b-9 can precisely guide complement modulation therapy for patients with severe trauma. Overall, this study suggests that targeting the right population of patients with the justified treatment at the right time would be a logical approach for complement modulation therapy after trauma. Cumulatively, this approach of personalized medicine will lead to better outcomes for trauma patients.
Acknowledgments
We thank the research nurses who supported this protocol, including Nancy Molter, Chaya Galin, Peggy Bielke, Kari Williams, Deb Deja, Elizabeth Frail, Juliette Castillo, and Michelle Morrow, and the information technologists, Nkolaos Kypreos and Jesus Morales, who assisted with database creation. The authors gratefully thank Dr Subrata Haldar for the revision and edition.
References
Footnotes
Presented at This study was presented as poster at the 2016 MHSRS (August 14–18, Kissimmee, Florida).
Contributors YL participated in the experimental design, performed measurement of sC5b-9 and functional complement pathway screening, data analysis and interpretation, and critical revision. QZ participated in literature search, data interpretation, and writing and formatting the article. BL conducted the assay of functional complement pathway screening. AD performed the data analysis. LC and MD provided critical revision. JDL participated in the experimental design, coordination, and article revision.
Funding This study was supported by a grant from US Army Medical Research and Materiel Command Combat Casualty Care Research Program (C_038_2014_USAISR).
Disclaimer The opinions or assertions expressed herein are the private views of the authors and are not to be construed as official or as reflecting the views of the US Department of the Army or the US Department of Defense.
Competing interests None declared.
Patient consent for publication Obtained.
Ethics approval The institutional review board of the US Army Medical Research and Materiel Command reviewed and approved the research protocol and granted a waiver of consent for the blood sampling as a minimal-risk intervention.
Provenance and peer review Not commissioned; externally peer reviewed.