Abstract
Background
Transcranial Doppler (TCD) pulsatility index (PI) has traditionally been interpreted as a descriptor of distal cerebrovascular resistance (CVR). We sought to evaluate the relationship between PI and CVR in situations, where CVR increases (mild hypocapnia) and decreases (plateau waves of intracranial pressure—ICP).
Methods
Recordings from patients with head-injury undergoing monitoring of arterial blood pressure (ABP), ICP, cerebral perfusion pressure (CPP), and TCD assessed cerebral blood flow velocities (FV) were analyzed. The Gosling pulsatility index (PI) was compared between baseline and ICP plateau waves (n = 20 patients) or short term (30–60 min) hypocapnia (n = 31). In addition, a modeling study was conducted with the “spectral” PI (calculated using fundamental harmonic of FV) resulting in a theoretical formula expressing the dependence of PI on balance of cerebrovascular impedances.
Results
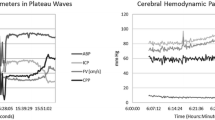
PI increased significantly (p < 0.001) while CVR decreased (p < 0.001) during plateau waves. During hypocapnia PI and CVR increased (p < 0.001). The modeling formula explained more than 65% of the variability of Gosling PI and 90% of the variability of the “spectral” PI (R = 0.81 and R = 0.95, respectively).
Conclusion
TCD pulsatility index can be easily and quickly assessed but is usually misinterpreted as a descriptor of CVR. The mathematical model presents a complex relationship between PI and multiple haemodynamic variables.




Similar content being viewed by others
References
Kontos HA. Validity of cerebral arterial blood flow calculations from velocity measurements. Stroke. 1989;20(1):1–3.
Gosling RG, King DH. Arterial assessment by Doppler-shift ultrasound. Proc R Soc Med. 1974;67(6 Pt 1):447–9.
Bellner J, Romner B, Reinstrup P, Kristiansson KA, Ryding E, Brandt L. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP). Surg Neurol. 2004;62(1):45–51.
Behrens A, Lenfeldt N, Ambarki K, Malm J, Eklund A, Koskinen LO. Transcranial Doppler pulsatility index: not an accurate method to assess intracranial pressure. Neurosurgery. 2010;66(6):1050–7.
Figaji AA, Zwane E, Fieggen AG, Siesjo P, Peter JC. Transcranial Doppler pulsatility index is not a reliable indicator of intracranial pressure in children with severe traumatic brain injury. Surg Neurol. 2009;72(4):389–94.
Melo JR, Di Rocco F, Blanot S, et al. Transcranial Doppler can predict intracranial hypertension in children with severe traumatic brain injuries. Childs Nerv Syst. 2011;27(6):979–84.
Soehle M, Chatfield DA, Czosnyka M, Kirkpatrick PJ. Predictive value of initial clinical status, intracranial pressure and transcranial Doppler pulsatility after subarachnoid haemorrhage. Acta Neurochir (Wien). 2007;149(6):575–83.
Giller CA, Hodges K, Batjer HH. Transcranial Doppler pulsatility in vasodilation and stenosis. J Neurosurg. 1990;72(6):901–6.
Lim MH, Cho YI, Jeong SK. Homocysteine and pulsatility index of cerebral arteries. Stroke. 2009;40(10):3216–20.
Czosnyka M, Richards HK, Whitehouse HE, Pickard JD. Relationship between transcranial Doppler-determined pulsatility index and cerebrovascular resistance: an experimental study. J Neurosurg. 1996;84(1):79–84.
Rosner MJ, Becker DP. Origin and evolution of plateau waves. Experimental observations and a theoretical model. J Neurosurg. 1984;60(2):312–24.
Steiner LA, Balestreri M, Johnston AJ, et al. Sustained moderate reductions in arterial CO2 after brain trauma time-course of cerebral blood flow velocity and intracranial pressure. Intensive Care Med. 2004;30(12):2180–7.
Hsu HY, Chern CM, Kuo JS, Kuo TB, Chen YT, Hu HH. Correlations among critical closing pressure, pulsatility index and cerebrovascular resistance. Ultrasound Med Biol. 2004;30(10):1329–35.
Kim DJ, Kasprowicz M, Carrera E, et al. The monitoring of relative changes in compartmental compliances of brain. Physiol Meas. 2009;30(7):647–59.
Czosnyka M, Smielewski P, Piechnik S, et al. Hemodynamic characterization of intracranial pressure plateau waves in head-injury patients. J Neurosurg. 1999;91(1):11–9.
Helmy A, Vizcaychipi M, Gupta AK. Traumatic brain injury: intensive care management. Br J Anaesth. 2007;99(1):32–42.
Lundberg N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr Scand Suppl. 1960;36(149):1–193.
Brain Trauma F, American Association of Neurological Surgeon, Congress of Neurological Surgeons, et al. Guidelines for the management of severe traumatic brain injury. XIV. Hyperventilation. J Neurotrauma 2007; 24 Suppl 1:S87–S90.
Raichle ME, Posner JB, Plum F. Cerebral blood flow during and after hyperventilation. Arch Neurol. 1970;23(5):394–403.
Czosnyka M, Richards HK, Reinhard M, et al. Cerebrovascular time constant: dependence on cerebral perfusion pressure and end-tidal carbon dioxide concentration. Neurol Res. 2012;34(1):17–24.
Czosnyka M, Piechnik S, Richards HK, Kirkpatrick P, Smielewski P, Pickard JD. Contribution of mathematical modelling to the interpretation of bedside tests of cerebrovascular autoregulation. J Neurol Neurosurg Psychiatry. 1997;63(6):721–31.
Carrera E, Steiner LA, Castellani G, et al. Changes in cerebral compartmental compliances during mild hypocapnia in patients with traumatic brain injury. J Neurotrauma. 2011;28(6):889–96.
Alperin N, Sivaramakrishnan A, Lichtor T. Magnetic resonance imaging-based measurements of cerebrospinal fluid and blood flow as indicators of intracranial compliance in patients with Chiari malformation. J Neurosurg. 2005;103(1):46–52.
Baledent O, Fin L, Khuoy L, et al. Brain hydrodynamics study by phase-contrast magnetic resonance imaging and transcranial color doppler. J Magn Reson Imaging. 2006;24(5):995–1004.
Michel E, Zernikow B. Goslig’s Doppler pulsatility index revisited. Ultrasound Med Biol. 1998;24(4):597–9.
Acknowledgment
This study was supported by the National Institute of Health Research, Biomedical Research Centre (Neuroscience Theme), the Medical Research Council (Grants G0600986 and G9439390), and NIHR Senior Investigator Awards (JDP); the Hospital Clinic Grant, Barcelona, Spain (NR) and also by the Swiss National Science Foundation (PBBSP3-125550 to CZ), Bern, Switzerland.
Disclosures
ICM+ Software is licensed by Cambridge Enterprise, Cambridge, UK, http://www.neurosurg.cam.ac.uk/icmplus/. MC and PS have a financial interest in a fraction of the licensing fee.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Mathematical Methods
The input circuit to cerebrovascular space (Fig. 5) can be reduced to cerebrovascular resistance (R a) and compliance (Ca) of arteries. Under the assumption that input pressure is low, and systolic–diastolic distance is totally contained within the range of autoregulation, the system may be treated as semi-linear.
Simplified input circuit used for the derivation of the formula (4) describing PI (calculated for fixed frequency of heart rate). a Input circuit representing a model of cerebral circulation. b Diagram of cerebrovascular impedance |Z(f)| as a function of frequency. Two frequencies are considered: f = 0 (i.e., DC component) and f = HR. Module of impedance for f = 0 is equal to R a, and may be estimated as CPPm/FVm. a1 pulse amplitude (first harmonic) of ABP, ABP arterial blood pressure, C a cerebrovascular compliance, CPPm mean cerebral perfusion pressure, f frequency, f1 pulse amplitude (first harmonic) of blood FV, FV m mean blood flow velocity in the middle cerebral artery, HR heart rate, PI Gosling pulsatility index, R a cerebrovascular resistance (in the main part of manuscript expressed as CVR), |z| cerebrovascular impedance, |z1| cerebrovascular impedance with frequency equal to HR
The pulsatility of the flow (PI), described as the ratio of the fundamental amplitude of the FV waveform divided by mean FV (1)
where f1 is the fundamental harmonic of FV, FVm is the mean FV, a1 is the fundamental harmonic of arterial pulse pressure, CPPm is the mean cerebral perfusion pressure, |z(0)| is the cerebrovascular impedance at zero frequency, |z(HR)| is the cerebrovascular impedance at frequency equal to heart rate.
Cerebrovascular impedance (Z(jω)) can be described as a complex function of frequency (2).
where ω is the 2πf, j is the imaginary unit, R a is the cerebrovascular resistance, Ca is the compliance of arteries and arterioles.
Z(f)| is described in (3)
where R a is the cerebrovascular resistance, Ca is the compliance of arteries and arterioles.
From here we can derive formula describing the pulsatility index:
where PI is the pulsatility index (for fundamental component of HR), a1 is the pulse amplitude (first harmonic) of ABP, CPPm is the mean cerebral perfusion pressure, R a is the cerebrovascular resistance, Ca is the compliance of arteries and arterioles, HR is the heart rate.
For the Gosling pulsatility index (PI) a1 should be substituted by the peak-to-peak amplitude of the ABP pulse. Also, Ca should be derived in a far more complex way, taking into account all harmonics of the ABP pulse and modules of impedances at these frequencies. But the general description of TCD pulsatility as a function of cerebral haemodynamic parameters remains the same.
Rights and permissions
About this article
Cite this article
de Riva, N., Budohoski, K.P., Smielewski, P. et al. Transcranial Doppler Pulsatility Index: What it is and What it Isn’t. Neurocrit Care 17, 58–66 (2012). https://doi.org/10.1007/s12028-012-9672-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-012-9672-6