Article Text
Abstract
Background Successful non-operative management (NOM) of blunt splenic trauma is enhanced with splenic angioembolization (SAE). Patients may still require splenectomy post-SAE for splenic infarction/necrosis. Prior studies have used white blood cell count (WBC), platelet count (PLT), and PLT:WBC ratio after splenectomy to predict complications, but none have evaluated these findings prior to splenectomy in patients who have undergone SAE. Changes in these values may indicate clinically significant splenic infarction, facilitating management of these patients.
Methods Patients admitted to an American College of Surgeons verified level 1 trauma center from January 2007 to August 2017 who underwent SAE were identified. Patients with successful NOM after SAE (SAE/NOM) were compared with those requiring splenectomy (SAE/SPLEN). Data included demographics, splenic injury grade, Injury Severity Score (ISS), time to SAE and splenectomy, intensive care unit and hospital length of stay (LOS), and complete blood count. Lab values were analyzed immediately post-SAE (time 1) and day 5 post-SAE (or day of discharge) for SAE/NOM patients and day of SPLEN for SAE/SPLEN patients (time 2). Data were analyzed using Mann-Whitney U, χ2 tests, and receiver operating characteristic (ROC) curves with significance attributed to P<0.05.
Results Of 124 patients undergoing SAE, 16 (13%) later required SPLEN for infarction/necrosis at a median of 5 days post-SAE (IQR: 3–10 days). SAE/SPLEN and SAE/NOM patients did not differ by age, gender, ISS, or grade of splenic injury. SAE/SPLEN patients had longer hospital LOS (23 vs. 10 days, P<0.001). WBC, PLT, and PLT:WBC ratio did not differ between the groups at time 1. At time 2, WBC was higher and PLT:WBC ratio was lower in SAE/SPLEN patients. Using ROC curves at time 2, the area under the curve was 0.90 (P<0.001) for WBC and 0.71 (P<0.007) for PLT:WBC ratio.
Discussion Patients requiring splenectomy for clinically significant infarction/necrosis after SAE develop leukocytosis and decreased PLT:WBC ratio when compared with SAE/NOM patients. Monitoring these parameters allows more prompt diagnosis and operative intervention.
Level of evidence Therapeutic/care management, level III.
- spleen injuries
- splenectomy
- leukocytosis
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Background
Successful non-operative management (NOM) of blunt splenic trauma has increased with the use of splenic angioembolization (SAE).1–6 The reported splenectomy rate for hemorrhage after SAE is about 3% to 22%.2 4 However, patients may still require splenectomy post-SAE due to symptomatic splenic infarction and necrosis. To our knowledge, this specific scenario has not been studied. Prompt recognition of these non-hemorrhagic complications can be challenging due to the non-specific symptoms of fever, abdominal pain, and tachycardia. Trends in white blood cell count (WBC), platelet count (PLT), and PLT:WBC ratio have been previously described for identifying patients with infection after splenectomy.7–9 However, no studies have evaluated symptomatic splenic infarction and necrosis using these findings prior to splenectomy in patients who have undergone SAE. We theorized that the alterations in these values may indicate clinically significant splenic infarction and thus facilitate the management of these patients.
Methods
Patients with blunt splenic injury admitted to Community Regional Medical Center, an American College of Surgeons-verified level 1 trauma center in Fresno, California, between 1 January 2007 and 31 August 2017, made up the study population. Patients were identified from the trauma registry and included if SAE was performed. Exclusion criteria were post-SAE splenectomy for hemorrhage, and preinjury splenomegaly or leukocytosis due to malignancy. Data included demographics, splenic injury grade, Injury Severity Score (ISS), time to SAE and splenectomy, intensive care unit and hospital length of stay (LOS), and laboratory studies. Complete blood count and PLT count values were noted immediately post-SAE, then daily, until discharge for SAE/NOM patients or splenectomy for SAE/SPLEN patients. Values immediately post-SAE were defined as time 1. Time 2 was defined as day 5 post-SAE (or day of discharge) for SAE/NOM patients and preoperatively on the day of splenectomy for SAE/SPLEN patients. Patients with successful NOM after SAE (SAE/NOM) were compared with those who required splenectomy for symptomatic infarction or necrosis (SAE/SPLEN). Patient records were reviewed for indications for splenectomy, operative findings, cultures of splenic tissue (if done), and pathologic reports. Postembolization CT scans were done at the discretion of the attending surgeon, and indications and results from these scans were reviewed.
Initial evaluation of appropriate, hemodynamically stable patients with blunt trauma was with contrast-enhanced CT scans. A 64-slice CT scanner (GE) was used at our institution between 2007 and 2013, and a 320-slice scanner (Toshiba) has been in use from 2013 to the present. SAE was performed if there was a visible splenic ‘blush’ or pseudoaneurysm visible on the admission CT. SAE was also performed for some patients with American Association for the Surgery of Trauma grade 4 and 5 splenic injuries per attending surgeon choice. SAE included both proximal and distal embolization. The technique for both proximal and distal SAE has been previously described in the literature.10 11
Statistical analyses were performed using the Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, V.23.0). Data were analyzed using Mann-Whitney U, Χ2 tests, and receiver operating characteristic (ROC) curves with significance attributed to P<0.05.
Results
During the 10.5-year study period, 40 591 patients were included in the trauma registry, with 30 974 having blunt mechanism (76%). Blunt splenic injury was identified in 1218 patients, 129 of whom underwent SAE. Of these, five were excluded: two due to post-SAE hemorrhage; one because of pretrauma splenomegaly and a WBC >100 000/µL due to leukemia; one patient had splenectomy over 6 months after SAE for a symptomatic, sterile splenic cyst; and one patient was excluded due to placement of a pigtail catheter in a splenic abscess by interventional radiology 1 month after initial discharge.
There were 124 patients included in the study, 108 with no intervention after SAE and 16 (13%) later requiring splenectomy for symptomatic infarction and necrosis, at a median of 5 days post-SAE (IQR: 3–10 days). SAE/SPLEN and SAE/NOM patients did not differ by age, gender, ISS, or grade of splenic injury (table 1). However, SAE/SPLEN patients had longer hospital LOS compared with SAE/NOM. There was no significant difference in the use of proximal versus distal embolization between the two groups (P=0.41; see table 1).
Patient characteristics
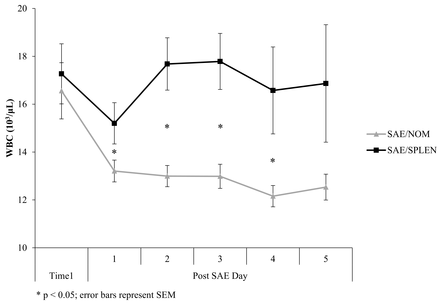
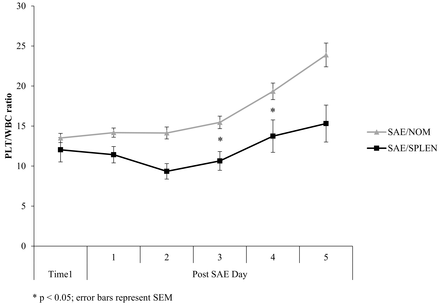
Comparisons of mean WBC and PLT:WBC are shown in figures 1 and 2. WBC, PLT, and PLT:WBC ratio did not differ between the groups at time 1. At time 2, WBC (103/μL) was significantly higher (22±6 vs. 12±4, P<0.001) and PLT:WBC ratio was significantly lower (16±7 vs. 23±15, P=0.007) in the SAE/SPLEN patients (table 2).
Patient outcomes
Comparison of white blood cell count (WBC). SAE/NOM, successful non-operative management after splenic angioembolization; SAE/SPLEN, splenectomy after splenic angioembolization.
Comparison of PLT:WBC. SAE/NOM, successful non-operative management after splenic angioembolization; SAE/SPLEN, splenectomy after splenic angioembolization; PLT:WBC, ratio of platelet count to white blood cell count.
ROC curves indicated that WBC had an area under the curve (AUC) of 0.90 (P<0.001) for predicting splenectomy (figure 3) with a threshold of >15 000/µL (sensitivity=0.81, specificity=0.81). The PLT:WBC ratio had an AUC of 0.71 (P=0.007) for predicting splenectomy, with an optimal threshold of <15 (sensitivity=0.75, specificity=0.69) (figure 4).
Receiver operating characteristic curve of white blood cell count for outcome of splenectomy.
Receiver operating characteristic curve of PLT:WBC ratio for outcome of splenectomy. PLT:WBC, ratio of platelet count to white blood cell count.
In all the patients who underwent splenectomy, unremitting abdominal pain, persistent fever, leukocytosis, and tachycardia were the primary reasons for post-SAE CT scanning. Significant splenic infarction and necrosis on post-SAE CT scan was an indication for operation. Seven of the SAE/SPLEN patients had moderate to large left pleural effusions. Five had small left pleural effusions. Four patients had no detectable left pleural effusion, three of whom had left chest tubes placed during their initial resuscitation. There were no documented concomitant or alternative intraoperative reasons for laparotomy documented in the SPLEN group, such as missed hollow visceral injuries or significant mesenteric injuries.
Review of the CT scans of the SAE/SPLEN patients demonstrated that all had at least 50% infarction or more of the spleen in all patients. The degree of infarction and necrosis was quantified on only 2 of the 16 CT radiology reports of the SPLEN patients (‘complete splenic infarction’ and ‘75% infarction’). Seven of the 16 (44%) of the SAE/SPLEN patients had air visualized within the parenchyma of the spleen on the postembolization CT scan. In the SAE/NOM patients, 12 of the 46 patients who had a postembolization CT scan (26%) had air noted within the spleen. All patients who had splenic air on CT had Gelfoam used in their SAE.
The pathologic reports of the removed spleens described unquantified degrees and combinations of the following: ‘hemorrhagic necrosis’, ‘inflammation’, ‘infarct’, ‘multiple infarcts’, ‘recent hemorrhage with necrosis’, ‘ischemia’, and ‘acute inflammation’. No cultures of the splenic tissue from the splenectomy specimens were performed.
Discussion
SAE has enhanced NOM of blunt splenic injuries,1–6 but non-hemorrhagic complications of infarction and necrosis of the spleen can still occur as a result.12–15 The SAE/NOM patients in our study represent a specific subset of patients who have non-specific symptoms such as fever and abdominal pain, as well as the associated findings of tachycardia and leukocytosis related to the splenic embolization. CT scan of the abdomen in these symptomatic, post-SAE patients will invariably demonstrate some degree of infarction of the spleen along with some areas of perfusion, as well as areas of air within the splenic parenchyma when Gelfoam is used.12–14 The purpose of our study was to investigate whether objective laboratory values could help with the management of symptomatic post-SAE patients whose splenic infarction has impelled clinical concern.
Splenectomy for symptomatic infarction and necrosis appears to occur infrequently, as it took over 10 years of chart review to obtain 16 cases. Although this complication is not common, it can be quite challenging to manage when it does occur, as this subset of SAE patients who neither immediately recover or progress to hemorrhage and urgent splenectomy appear to ‘smolder’ in the hospital. Fevers, tachycardia, ileus, concerning leukocytosis, or unremitting abdominal discomfort appear to plague this group of patients who fails to definitively recuperate from blunt splenic injury after embolization.
WBC count and PLT count have been used to help differentiate between infection and the normal leukemoid response to splenectomy.7–9 Using these same parameters seemed to be a logical step in the management of post-SAE patients. Specific CT findings after splenic embolization, including infarcts, have been described in 63% of patients after proximal embolization and up to 100% after distal embolization.12–14 However, the specific clinical context, such as symptoms or clinical findings, has not been defined. The studies by Ekeh et al13 and Wu et al14 also describe CT findings after SAE but without detailing clinical findings or symptoms related to each of the scans. Haan et al1 described low-grade fever or left upper quadrant pain as ‘minimal symptoms’ in SAE/NOM patients seen in follow-up who were treated expectantly with analgesics and two patients with ‘large, symptomatic’ splenic infarcts with air on follow-up CT who required splenectomy. Cultures taken from the two removed spleens at time of operation grew no organisms.1
Our study focused on inpatients who had clinically significant and symptomatic post-SAE infarction and necrosis. Weng et al and Toutouzas et al7 9 found that postoperative day 5 after splenectomy was the mean time that patients with documented infection had a significant increase in their WBC to >15 000 and decrease in PLT:WBC ratio <15. In the present study, the median day of splenectomy after angioembolization was day 5 in the SAE/SPLEN group (time 2). Time 2 was therefore designated as day 5 in the SAE/NOM patients. Some of our NOM patients were discharged sooner than post-SAE day 5; thus, data from the day of discharge were included as time 2 for these SAE/NOM patients.
Review of the CT scans of our SAE/SPLEN patients demonstrated that all had at least 50% infarction of the spleen. Haan et al1 defined a significant infarction as greater than 25% of the spleen. In the present study, the degree of infarction and necrosis was quantified on only 2 of the 16 CT radiology reports of the SPLEN patients (‘complete splenic infarction’ and ‘75% infarction’). Most of the reports used qualitative terms such as ‘extensive infarct’, ‘extensive necrosis’, and ‘diffuse infarction’. There was no significant difference in the use of proximal versus distal embolization technique between the two groups. The systemic review and meta-analysis by Schnüriger et al15 also noted that both techniques had an equivalent rate of major infarctions and infections requiring splenectomy.
Our study supports the use of WBC greater than 15 000 in symptomatic post-SAE patients who have ongoing abdominal pain, fever, and tachycardia as an indication for splenectomy. The AUC analysis demonstrates WBC threshold greater than 15 000 (sensitivity=0.81, specificity=0.81) and PLT:WBC ratio threshold less than 15 (sensitivity=0.75, specificity=0.7) as the peak areas of each curve, respectively. However, WBC appeared to be the more sensitive and specific marker of symptomatic splenic infarction requiring splenectomy. Although a WBC of 15 000 is at the ‘peak’ of the ROC curve, a WBC of greater than 17 000 has a specificity of about 90% and therefore a lower false-positive rate compared with 15 000.
PLT count by itself did not appear to have a useful correlation in helping define SAE patients who would require splenectomy. Increasing WBC (the denominator) is the driving force that decreases the PLT:WBC ratio, not the decrease in PLT count. This result is similar to that in the study by Banerjee et al,8 in which splenectomy patients who developed postoperative infection had a lack of significant PLT elevation.
The presence of intraparenchymal splenic air after embolization with Gelfoam has been previously described.9 The fact that both groups had air noted in the spleen supports the argument that it was NOT the presence of gas in the spleen on CT per se that compelled the operation but the degree of leukocytosis and infarction.
This study is prone to the weaknesses of retrospective reviews, including the fact that attending surgeons were not blinded to the laboratory values (WBC, PLT) analyzed in this investigation, and these observed results were included in the decision to perform splenectomy.
In conclusion, a small proportion of patients with blunt splenic injury who undergo SAE will require splenectomy for clinically important splenic infarction/necrosis. These patients have significant leukocytosis and decreased PLT:WBC ratio when compared with patients with successful SAE/NOM. Post-SAE patients who have persistent tachycardia, abdominal pain, fever, WBC greater than 15 000, and/or PLT:WBC ratio less than 15 should get a CT scan of the abdomen and are likely to require splenectomy for resolution of symptoms. Monitoring these parameters allows more prompt diagnosis and operative intervention.
References
Footnotes
Contributors JFB: study design, literature search, data collection, data analysis, data interpretation, writing, critical revision. VLS: study design, literature search, data collection, data interpretation. RCD: study design, data collection, data analysis, data interpretation. KLK: data analysis, data interpretation, critical revision. JWD: data analysis, data interpretation, critical revision.
Funding This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Ethics approval This study was approved by the Institutional Review Board for Community Medical Centers and the University of California, San Francisco-Fresno.
Provenance and peer review Not commissioned; externally peer reviewed.