- Benjamin M Howard1,
- Lucy Z Kornblith1,
- Sabrinah A Christie1,
- Amanda S Conroy1,
- Mary F Nelson1,
- Eric M Campion2,
- Rachael A Callcut1,
- Carolyn S Calfee3,
- Brandon J Lamere4,
- Douglas W Fadrosh4,
- Susan Lynch4,
- Mitchell Jay Cohen2
- 1 Department of Surgery, San Francisco General Hospital, University of California San Francisco, California, USA
- 2 Department of Surgery, Denver Health and Hospital Authority, University of Colorado, Denver, Colorado, USA
- 3 Division of Pulmonary and Critical Care Medicine, Departments of Medicine and Anesthesia, University of California San Francisco, California, USA
- 4 Division of Gastroenterology, Department of Medicine, University of California San Francisco, California, USA
- Correspondence to Dr Benjamin M Howard, Department of Surgery, Ward 3A San Francisco General Hospital, 1001 Potrero Avenue, Room 3C-38, San Francisco, CA 94110, USA; benjamin.howard{at}ucsf.edu
- Received 3 May 2017
- Revised 15 June 2017
- Accepted 26 June 2017
Abstract
Background Recent studies have demonstrated the vital influence of commensal microbial communities on human health. The central role of the gut in the response to injury is well described; however, no prior studies have used culture-independent profiling techniques to characterize the gut microbiome after severe trauma. We hypothesized that in critically injured patients, the gut microbiome would undergo significant compositional changes in the first 72 hours after injury.
Methods Trauma stool samples were prospectively collected via digital rectal examination at the time of presentation (0 hour). Patients admitted to the intensive care unit (n=12) had additional stool samples collected at 24 hours and/or 72 hours. Uninjured patients served as controls (n=10). DNA was extracted from stool samples and 16S rRNA-targeted PCR amplification was performed; amplicons were sequenced and binned into operational taxonomic units (OTUs; 97% sequence similarity). Diversity was analyzed using principle coordinates analyses, and negative binomial regression was used to determine significantly enriched OTUs.
Results Critically injured patients had a median Injury Severity Score of 27 and suffered polytrauma. At baseline (0 hour), there were no detectable differences in gut microbial community diversity between injured and uninjured patients. Injured patients developed changes in gut microbiome composition within 72 hours, characterized by significant alterations in phylogenetic composition and taxon relative abundance. Members of the bacterial orders Bacteroidales, Fusobacteriales and Verrucomicrobiales were depleted during 72 hours, whereas Clostridiales and Enterococcus members enriched significantly.
Discussion In this initial study of the gut microbiome after trauma, we demonstrate that significant changes in phylogenetic composition and relative abundance occur in the first 72 hours after injury. This rapid change in intestinal microbiota represents a critical phenomenon that may influence outcomes after severe trauma. A better understanding of the nature of these postinjury changes may lead to the ability to intervene in otherwise pathological clinical trajectories.
Level of evidence III
Study type Prognostic/epidemiological
- microbiome
- critical illness
- inflammatory response
- trauma and sepsis
Background
While microbial organisms have long held the interest of surgeons in general and critical care specialists in particular, a proliferation of recent research has changed our conception of the relationship between human hosts and their indwelling microbiomes.1 2 Improved ability to characterize microbial communities using culture-independent DNA sequencing techniques has led to an increased awareness of the vital influence of such communities on human health and disease states, both acute and chronic.3 4 Such investigations have focused on particular demographics and anatomic regions, with resulting findings that have led to paradigm shifts in the way long-studied diseases are understood and treated.5–10
The central role of the gut in the response to injury has been well described,11 and major alterations in gut physiology and flora have been associated with critical illness.12–14 Though alterations in gut function have been associated with the sterile inflammation that characterizes the critical illness state after injury,15 16 no prior studies have used culture-independent profiling techniques to characterize the gut microbiome in a cohort of patient with severe multisystem trauma. Early culture-independent investigations of gut flora in intensive care unit (ICU) patients would suggest that microbial composition at the time of admission may correlate to critical outcomes17; indeed, investigators have even reported resolution of multiple organ dysfunction syndrome secondary to sepsis by treating with fecal transplant, demonstrating that clinical improvement and reduced inflammation was associated with reconstitution of the gut microbiome.18 However, to our knowledge, no studies have conducted serial evaluations at multiple time points specifically in a trauma population. As such, it remains difficult to derive conclusions as to the causal implications of such changes, and their importance in a traumatically injured patient.
Given the lack of previous data describing the gut microbiome after severe trauma, we sought to better characterize the changes that occur in critically injured patients using culture-independent DNA sequencing techniques. Our aim was to describe any baseline differences in microbial community in the severely injured, to highlight changes in composition that occur after initial resuscitation and during the early days of ICU stay, and to correlate such changes to demographic characteristics and clinical interventions. Our long-term goal was to establish a better foundational understanding of microbiome dynamics after trauma, to serve as a basis for future investigation and possible therapeutic intervention.
We hypothesized that in critically injured patients, the gut microbiome would undergo significant compositional changes in the first 72 hours after injury. We further hypothesized that these changes would occur after initial evaluation and resuscitation, and thus that microbial community composition would not differ significantly between severely injured patients and uninjured control patients at time of arrival.
Methods
Data were prospectively collected from patients with trauma who presented at a single urban level 1 trauma center from 2014 to 2015, under a protocol approved by the University of California, San Francisco Committee on Human Research (CHR). Patients’ stool samples were collected via digital rectal examination at the time of presentation in the emergency department (0 hour). Patients who met criteria for highest level critical trauma activation were eligible for inclusion in the study. For injured patients who required admission to the ICU (n=12), additional stool samples were collected at 24 hours and/or 72 hours, with informed consent obtained from the patient or their medical decision maker in accordance with the CHR-approved protocol. All samples had to yield visible amounts of stool to be included; if no visible stool was present at the time of presentation (0 hour), patients were not included in the study. If patients were transferred out of the ICU to lower acuity units prior to 72 hours after admission, they were excluded from analysis. Thus, patients who were injured but not admitted to the ICU for at least 72 hours were excluded from analysis. Patients with isolated traumatic brain injury were excluded. Patients with activated trauma found to be uninjured, with Injury Severity Score (ISS) of 0 or 1 and length of stay less than 1 day, served as controls (n=10). Comprehensive demographic, injury, clinical and outcomes data were prospectively collected on all included patients.
Initial stool samples were collected by digital rectal examination performed by the examining trauma or emergency room physician at the time of secondary evaluation in the trauma resuscitation room, as part of the standard trauma examination. Subsequent ICU stool samples were collected either by digital rectal examination, or in passed stool if patients had a bowel movement at the time of planned sample collection. Samples were collected on a sterile glove, which was then taken to the lab and placed in a freezer maintained at −80°C within 20 minutes of collection time; the collecting finger of each glove was detached, inverted and placed in a sterile collection tube.
DNA extraction, PCR and DNA sequencing were performed on the Illumina MiSeq gene sequencer (Illumina Inc., San Diego, CA). DNA was extracted from stool samples using the MO BIO PowerSoil DNA Isolation Kit (MO BIO Laboratories Inc., Carlsbad, CA) and Qiagen AllPrep DNA/RNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations, respectively. Each DNA sample was PCR amplified in triplicate using primer pairs that (1) targeted the V4 hypervariable region of the 16 s rRNA gene, (2) contained a unique bar code sequence to enable demultiplexing of pooled samples and (3) contained an adapter sequence enabling the amplicon to bind to the MiSeq flow cell. Successful amplicons (50 of 51 samples, 98%) were pooled in equal molar concentrations and sequenced on the Illumina MiSeq.
Paired sequencing reads were quality filtered and demultiplexed using the QIIME software package before being assembled and processed further. Briefly, assembled sequencing read pairs were binned into operational taxonomic units (OTUs) using a 97% similarity to the Greengenes database, and reads that either did not cluster to the Greengenes database or that were chimeric were removed from subsequent analyses. Sample read numbers were rarefied to the read number of the lowest usable sample after processing (44 372), resulting in a rarefied OTU table.
Measures of alpha-diversity, or overall community composition within a tested population (in this case each patient at the specified time point), were derived, with richness (number of different OTUs), evenness (how equal the abundances of the OTUs are) and diversity (Shannon, Simpson and Inverse Simpson) assessed for each comparison group (samples at 0 hour, 24 hours, 72 hours). Beta-diversity, which measures differences in community composition between samples, was calculated and analyzed using principle coordinates analyses, with the derivation of Curtis, Canberra, weighted UniFrac and unweighted UniFrac distance matrices. Negative binomial regression was used to determine significantly enriched OTUs. An alpha of 0.05 was considered significant. All sample processing and subsequent analysis was performed by the authors.
Results
Patients who met inclusion criteria were predominantly male, with an average age of 49 years (table 1). Included patients were severely injured, with median ISS of 27 and mean base deficit −6.1 mEq/L; all patients suffered polytrauma, or traumatic injuries to multiple systems, and most had blunt mechanisms of injury.
Patient demographics, injury characteristics, treatments and outcomes
Patients included as controls in analysis all had ISS of 0 or 1, and were either discharged from the emergency department or from the inpatient ward within 24 hours of admission. There were no significant differences in age, gender or ethnicity between these uninjured control patients and the cohort of severely injured patients.
As shown in table 2, there were no significant differences in stool microbiome alpha-diversity between uninjured control patients and severely injured patients at time of admission.
Measures of alpha-diversity in stool microbiome (p Values)
Similarly, there were no differences in beta-diversity indices between these two groups (table 3), indicating that injured and uninjured patients share comparable microbial community composition at the time of admission.
Measures of beta-diversity in stool microbiome (p Values)
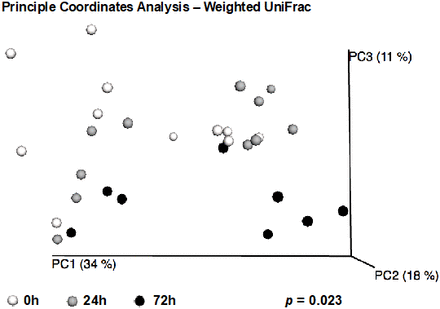
Though overall alpha-diversity measures did not change between time points among injured patients, these patients went on to develop significant changes in gut microbiome composition within 72 hours, as measured by beta-diversity. A significant difference between the microbial community composition of critically injured patients from admission to 72 hours after admission was observed when analyzing the weighted UniFrac matrices, indicating both a phylogenetic composition and taxon relative abundance component to the difference between the microbial communities (weighted UniFrac p=0.023, figure 1).
Beta-diversity changes during 72 hours. A significant difference between the microbial community composition of critically injured patients from admission (0 hour), 24 or 72 hours after admission was observed when analyzing the weighted UniFrac matrices, as depicted in three-dimensional Principle Coordinates Analysis. This implies that there is both a phylogenetic and relative abundance component to the difference between the microbial communities.
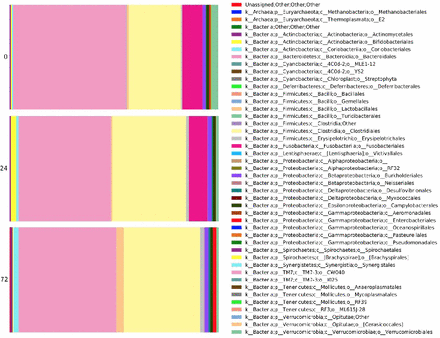
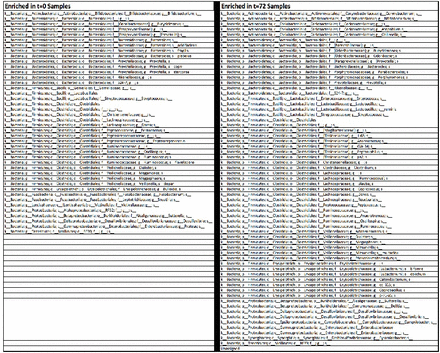
To better understand such differences, changes in significantly enriched OTUs across time points were analyzed. Changes in relative order abundance are depicted in figure 2. This analysis revealed that members of the bacterial orders Bacteroidales, Fusobacteriales and Verrucomicrobiales were depleted during 72 hours, whereas Clostridiales and Enterococcus members enriched significantly (negative binomial regression p<0.05). Overall, there were 124 significantly enriched OTUs at 0 hours, and a distinct 151 significantly enriched OTUs at 72 hours. These order-level changes are depicted in figure 3.
Microbial changes over time, depicted at order taxonomic level.
Significantly enriched OTUs at 0 vs. 72 hours. Significantly enriched OTUs (p<0.05, q<0.05) were determined using negative binomial regression. When comparing the 0 and 72 hours samples there were 124 and 151 significantly enriched OTUs at each time point, respectively. OTU, operational taxonomic units
Discussion
Through novel methods of characterizing microbial community composition, an enhanced understanding of the relationship between commensal organisms and human health is emerging across medical specialties and scientific disciplines. This understanding has led to promising diagnostic and even therapeutic modalities; perhaps the most widely recognized application is that of fecal transplant in colitis due to Clostridium difficile.19 Still, our understanding of such processes remains in its incipient stages, and has yet to be thoroughly examined in trauma populations. Culture-based studies have indicated that critical illness may correlate to significant alterations in microbial populations,13 a view that has been supported by increasing numbers of culture-independent investigations.14 20 One recent study of burn injury in both animal models and humans suggests that significant restructuring of the microbiome occurs after burn, and may contribute to increased rates of clinical sepsis.21 A recent case series demonstrated that in severe burn victims, initially pathogenic changes in the gut microbiota were reversed in survivors, indicating an association between regeneration of ‘healthy’ microbial communities with survival.22 Another animal investigation demonstrated that altering the gut microbiome led to significant changes in inflammatory responses to remote organ injury (specifically, ischemia reperfusion in lung), thus implicating intestinal microbiota as critical in the response to sterile inflammatory injury.23 Fecal transplant in murine mouse model has been shown to restore mucosal integrity,24 which has been posited by others as a possible strategy for preventing gut translocation and associated sepsis in surgical patients who are critically ill.25 The emerging understanding of the relationship between the gut microbiome and inflammation26–28 represents an area of clear relevance and critical importance to trauma and critical care. To date, however, no dedicated study has prospectively investigated changes in the microbiome in a critically injured trauma population.
In this first prospective study of the gut microbiome after trauma, we demonstrate that significant changes in microbial phylogenetic composition and relative abundance occur in the first 72 hours after injury. Such changes are not apparent at the time of initial assessment, indicating that these patients are no different from controls upon presentation; their injury patterns and subsequent therapeutic interventions are thus correlated to their microbial compositional shifts, via a yet-to-be-determined causal relationship. The short time course in which such alterations occur is also notable—such relatively rapid alterations in intestinal microbiota represent a critical and previously unrecognized phenomenon that may influence clinical course and outcomes after severe trauma.
The main strength of this study is its ability to combine comprehensive clinical characterization of a severely injured trauma population with high-throughput culture-independent techniques for characterizing microbial community composition. As a pilot investigation, this study has limitations, the most critical of which is sample size. Though all critical trauma activation patients were initially eligible for inclusion, the number who had visible stool on initial digital rectal examination and who remained in the ICU for 72 hours constituted a small subset. Our findings here present the initial analysis of a growing cohort, and must be interpreted with the number of included patients in mind. Several critical questions, especially those regarding our initial secondary hypotheses as to clinical course and outcomes, will require significantly more patients before any definitive conclusions can be drawn. In particular, the known influence of antibiotics on diversity changes should be analyzed given our initial findings in this cohort, and should be extrapolated from the influence of injury independent of antimicrobial administration. This represents an area of ongoing investigation in our group. Also, though data in this study were collected prospectively, the findings in this cohort should be interpreted as correlative and not necessarily causal. In addition, the finding of significant beta-diversity differences was identified by one analytic approach (weighted UniFrac) but not others; future studies would of course be strengthened if multiple analyses yielded statistically significant results.
The gut bacterial community is known to modulate inflammation, and is related to a range of clinical outcomes in the patient who is critically ill. Our findings of rapid microbiome changes after severe injury indicate that commensal microbial populations undergo significant changes early in the course of resuscitation and stabilization after trauma. The correlation of microbiota composition to clinical features, course and outcomes represents an area of active ongoing research in our lab, and may represent an area in which the care of the injured patient might be optimized. Implementing a probiotic regimen or guiding the microbial composition changes after trauma might prove a powerful tool in the critical care arsenal, and remains an area of active investigation in our lab and others.29–31 Though causal relationships remain to be determined, a better understanding of the nature of these postinjury changes may lead to the ability to intervene in otherwise pathological clinical trajectories.
Acknowledgments
The authors thank the nurses, physicians and house staff of San Francisco General Hospital for their assistance in making this study possible.
Footnotes
Initial findings presented at the annual meeting of the American Association for the Surgery of Trauma, Las Vegas, Nevada, September 2015.
Contributors All authors contributed to study design, sample and data collection, data analysis, or writing and revision, or a combination thereof.
Competing interests None declared.
Ethics approval UCSF Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/