Article Text
Abstract
Many trauma systems are examining whether to implement prehospital tranexamic acid (TXA) protocols since hemorrhage remains the leading cause of potentially preventable early trauma mortality, and early in-hospital administration of TXA within 3 hours of injury is associated with reduced mortality. But robust evidence regarding the efficacy of prehospital administration of the antifibrinolytic drug TXA on trauma outcomes is lacking. This review examines the current evidence available regarding prehospital TXA efficacy in both military and civilian trauma, and updates available evidence regarding in-hospital TXA efficacy in trauma.
- tranexamic acid
- Prehospital care
- hemorrhage
- fibrinolysis
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Many trauma centers are examining the potential use of tranexamic acid (TXA) in the prehospital phase of care in an attempt to provide an adjunct to early hemorrhage control to improve trauma outcomes. But the published evidence on prehospital TXA use in trauma is small. This review, therefore, first updates the current evidence regarding in-hospital TXA efficacy and examines the evidence for efficacy of prehospital use of TXA in trauma. These data are needed to determine whether to implement prehospital TXA protocols or not, and to determine which trauma patients may benefit from prehospital TXA administration for prehospital protocol development.
Evidence for in-hospital TXA
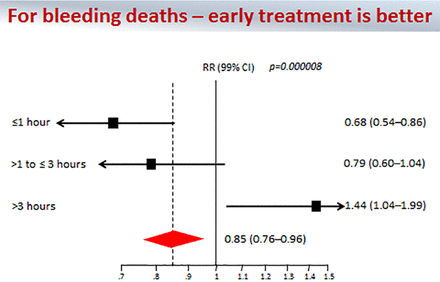
At present, there is still only one large randomized clinical trial (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage 2 (CRASH-2)) that examined the efficacy of in-hospital TXA in trauma and documented that all-cause mortality was reduced from 16.0% to 14.5% (1.5% absolute reduction, RR 0.91, 95% CI 0.91 (0.85 to 0.97), p=0.0035, NNT 67) and risk of death caused by bleeding was reduced from 5.7% to 4.9% (0.8% reduction, NNT 121).1 Importantly, for bleeding deaths, early TXA treatment was better: TXA given ≤1 hour after injury was more protective than when given 1–3 hours after injury, and TXA given after 3 hours was associated with increased risk of death (figure 1).
CRASH-2 trial results; RR all-cause in-hospital mortality based on timing of TXA administration. Early TXA (≤1 hour from injury) is associated with survival benefit. From Shakur et al.1
We have previously reviewed the significant limitations of the CRASH-2 trial and the difficulty in translating the international study results to large civilian trauma centers and trauma systems of care.2 We also reviewed that in the CRASH 2 subgroup analysis, the most significant mortality benefit for TXA was in the severe shock cohort, trauma patients with admission systolic blood pressure (SBP) ≤75 mm Hg with 28-day all-cause mortality of 30.6% for TXA vs 35.1% for placebo (RR 0.87, 99% CI 0.76 to 0.99).
TXA use (administered to patients receiving at least 1 unit of packed red blood cells) was associated with improved survival (OR 7.3; 95% CI 3.02 to 17.32) in battle casualties in the military setting from a Role 3 Echelon combat surgical hospital in southern Afghanistan (retrospective observational studies, Military Application of Tranexamic Acid in Trauma Emergency Resuscitation, MATTERs).3 ,4
What civilian trauma data are available regarding TXA impact on trauma outcomes? Three civilian single-center studies from large urban trauma centers examined the impact of implementation of a protocol for in-hospital TXA administration on trauma outcomes.
A single-center study (Ryder Trauma Center, Miami, Florida) examined patients who were under emergency surgical intervention directly from ED resuscitation area or required blood transfusions (n=1217, 8/2009–1/2013). TXA was initiated on 3/2011 at surgeon discretion. TXA patients (n=150) were matched to controls with propensity scores using the variables of age, sex, traumatic brain injury (TBI), mechanism of injury, SBP, blood transfusion and injury severity score (ISS). For the highest injury acuity patients, TXA was associated with increased mortality (27% vs 17%, p=0.024). The authors stated that, in most patients, TXA was administered after the patient had already received transfusion of blood products. The lack of benefit of TXA may be related to the rapid availability of blood product resuscitation in mature trauma centers such as this.5
A single-center study (University of Texas Health Science Center-Houston) reported their implementation of a protocol to administer TXA in trauma patients with evidence of hyperfibrinolysis (defined as LY-30 of 3% or greater by rapid thromboelastography) on admission in 2011. Trauma registry data for all adult patients from 9/2009 to 9/2013 with evidence of hyperfibrinolysis (n=1032) were examined. Unadjusted in-hospital mortality was higher in the TXA group (40% vs 17%, p<0.001). Using logistic regression analysis (controlling for age, sex, ISS, arrival physiology and base deficit) TXA did not reduce in-hospital mortality (OR 0.74; 95% CI 0.38 to 1.40; p=0.80) in patients with documentation of viscoelastic hyperfibrinolysis.6
Another single-center prospective cohort study (Queen Mary University, London, UK) examined the impact of TXA use on trauma outcomes in severely injured civilian patients as they implemented their institutional protocol for TXA into the hospital's massive transfusion protocol (MTP). The TXA protocol was administration either in the prehospital care phase or the ED if the SBP was <90 mm Hg, there was poor response to an initial fluid bolus and there was suspected active hemorrhage. This study retrospectively excluded patients found to have an ISS <15. Of a total cohort of 385 patients, 160 (42%) received TXA within 3 hours of injury in either prehospital or ED phase of care. But data regarding how many patients received TXA prehospital was not provided. Patients who received TXA were more severely injured, shocked and coagulopathic on arrival. Patients were further separated into ‘shock’ (defined as BD ≥6 mEq/L) versus ‘non-shock’ (BD <6 mEq/L) groups for further analysis. TXA was not independently associated with any change in the outcome for either the overall or non-shock cohorts. In multivariate analysis, TXA was independently associated with a reduction in multiple organ failure (MOF, OR 0.27, CI 0.10 to 0.73, p=0.01) and was protective for adjusted all-cause mortality (OR=0.16 CI 0.03 to 0.86, p=0.03) only in the ‘shock’ patients. No difference in 48 hour mortality was identified in the shock cohort.7 This study concluded “it is difficult to recommend TXA use in nonshock patients within mature civilian trauma systems”.
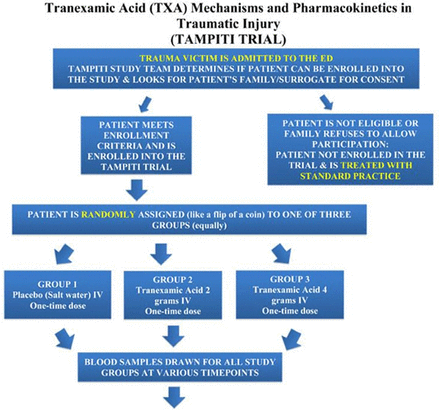
A clinical trial of in-hospital TXA use (TAMPITI, Tranexamic Acid (TXA) Mechanisms and Pharmacokinetics in Traumatic Injury, figure 2) is ongoing at Barnes-Jewish Hospital, St Louis. This study (funded by the Department of Defense) is randomizing adult trauma patients (n=150 planned) ordered to receive at least 1 blood product and/or immediate transfer to operating room to control bleeding, and within 2 hours of injury to either (1) placebo, (2) TXA 2 g intravenously or (3) TXA 4 g intravenously. The primary outcome measure is immune parameters and monocyte function at 0–72 hours and pharmacodynamics, aiming to examine potential mechanisms by which TXA may be beneficial in trauma.8 ,9
TAMPITI trial of in-hospital TXA. From http://www.tampiti.wustl.edu/.
Surveys regarding TXA use in trauma
An online survey of surgeons in US civilian trauma centers of the Eastern Association for the Surgery of Trauma (EAST) reported that TXA was available at 89.1% of centers but that the use of TXA was extremely variable. Only 38% of respondents use TXA regularly for significant traumatic hemorrhage. Reasons for not routine TXA use included uncertain clinical benefit (47.7%) and unfamiliarity (31.5%). Most respondents (90.5%) indicated that they desire national organizations to develop practice guidelines. In this survey, a majority (78.4%) of respondents agreed that TXA has a role in civilian prehospital care.10
In a survey of 125 level I and II trauma centers conducted via the American Association for the Surgery of Trauma (AAST), nearly all (98.4%) of trauma centers have an MTP. TXA is part of the MTP in 64% of trauma centers and only 9% routinely use thromboelastography or rotational thromboelastometery (TEG/ROTEM) within their MTP.11
Fibrinolysis phenotypes: rationale for selective TXA administration
Concern for early TXA use in all severely injured patients may in part be related to findings of a hypofibrinolysis (shutdown) phenotype. The use of viscoelastic assays in trauma has identified three distinct phenotypes: (1) hyperfibrinolysis (LY30 ≥3%), (2) physiologic (LY30 0.81–2.9%) and (3) hypofibrinolysis (shutdown, LY30 ≤0.08%).12 In a single-center study of 180 severely injured patients with median ISS 29 (IQR 22–36), median initial BD 9 and mortality 20% (2/3 within 24 hours), it was identified that hypofibrinolysis (shutdown) was the most common phenotype identified in 64% of patients, with physiologic and hyperfibrinolysis identified in 18% each. Mortality had a U-shaped distribution with 44% in the hyperfibrinolysis group, 17% in the hypofibrinolysis patients and 3% in the physiologic group. Acute blood loss accounted for 66% of the mortality in the hyperfibrinolysis group compared with 15% in the fibrinolysis shutdown patients. Conversely, death because of MOF occurred in 7% of the hyperfibrinolysis group compared with 40% in the shutdown patients.13
A follow-up study from two institutions included 2540 severely injured patients in a similar analysis, with median age 39 years, median ISS 25 and mortality rate 21%, and confirmed the previous study findings. Fibrinolysis shutdown was the most common phenotype (46%) followed by physiologic (36%) and hyperfibrinolysis (18%). Hyperfibrinolysis was associated with the highest death rate (34%), followed by shutdown (22%), and physiologic (14%, p<0.001). The risk of mortality remained increased for hyperfibrinolysis (OR 3.3, 95% CI 2.4 to 4.6, p<0.0001) and shutdown (OR 1.6, 95% CI 1.3 to 2.1, p=0.0003) compared with physiologic when adjusting for age, ISS, mechanism, head injury and blood pressure (AUROC 0.82, 95% CI 0.80 to 0.84). The authors concluded that these data provide additional evidence of distinct phenotypes of coagulation impairment after trauma and that individualized hemostatic therapy may be required.14
Evidence for prehospital TXA in civilian trauma
One retrospective German study examined trauma outcomes related to prehospital TXA treatment.15 Linking data from a prehospital database and trauma registry from 2012 to 2014, a cohort of patients who received TXA prehospital, were compared to a propensity score-based matched pairs cohort (n=258 in each group). TXA was provided by 20 of the 35 air rescue helicopters during the 3-year study period. The majority of patients (90%) had sustained blunt trauma and had a mean ISS of 24. Early mortality was significantly lower in the TXA cohort (6 hour mortality 1.9 vs 9.3%, p<0.001; 12 hour mortality 3.5% vs 10.9%, p=0.002; 24 hour mortality 5.8% vs 12.4%, p=0.01). Overall in-hospital mortality was similar in both groups (14.7% vs 16.3%; p=0.72). The mean time to death was 8.8±13.4 days vs 3.6±4.9 days, respectively (p=0.001). This first civilian study confirmed that TXA was associated with significantly improved early survival and prolonged time to death.
But a number of limitations of this study are recognized which limit its generalizability:
Exact timing of prehospital TXA administration and dosages were not documented,
TXA administration at discretion of emergency physician, no standardized algorithm,
Cause of death was not documented, therefore deaths due to hemorrhage versus TBI are unknown, cannot determine whether TXA was associated with reduced mortality due to hemorrhage.
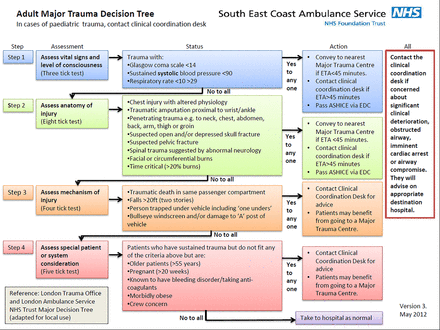
The UK London Ambulance Service has used prehospital TXA in the civilian setting the longest with the following protocol: “Patients with time critical injury where significant internal/external hemorrhage is suspected and/or injured patients >12 years of age, fulfilling local Step 1 or Step 2 on the major trauma decision tree” (figure 3). But data regarding efficacy of prehospital TXA using this protocol are lacking.
UK London ambulance service prehospital TXA protocol. Patients with time critical injury where significant internal/external hemorrhage is suspected and/or injured patients >12 years of age, fulfilling local Step 1 or Step 2 on the major trauma decision tree.
Evidence for prehospital TXA in military trauma
A prehospital TXA treatment protocol was approved by the Israel Defence Forces Medical Corps (IDF-MC) for all advanced life support providers in 5/2011. A review of outcomes in 40 patients who received intravenous TXA prehospital from 12/2011 to 2/2013 reported a mortality rate of 17% but no comparison cohort. Interestingly, 30% of patients had no clear indication for TXA administration based on the IDF TXA protocol, and mortality was 0% in those patients, vs 23% in patients with TXA indicated.16 An additional IDF-MC report of 258 casualties on the Syrian border confirmed that TXA was administered in 30% (n=47) of the patients, the majority (85%, n=40) of whom had penetrating injury and tachycardia (65%, n=28).17 But efficacy of TXA cannot be determined in these case series.
The Israeli National Civilian emergency medical service (EMS) system initiated modified prehospital TXA protocol in 11/2011, but the military and civilian protocols are slightly different (table 1). To the best of our knowledge, the Israeli National EMS is the only nationwide prehospital civilian response service to administer TXA. The combined report of 103 casualties who received prehospital TXA documented an overall mortality rate of 18% with median ISS of 16, and confirmed that TXA administration in the field is feasible.18
Comparison of TXA administration protocols between the IDF and civilian EMS in Israel (from Nadler et al18)
The USA, French, British and Israeli militaries and British, Norwegian, Israeli civilian ambulance services have implemented TXA use as part of RDCR policies in the scenario of prolonged time to hospital. Policies for the use of TXA in the prehospital setting have been implemented since 2010 by the British Army medical emergency response teams, since 2011 by the French army, the NHS ambulance service in the UK, the British Columbia Ambulance Service's AirEvac and Critical Care Operations civilian service (report of 13 patients over 4 months19) and a civilian air ambulance service in Bergen Norway.
Pre-hospital TXA guidance
TXA was added to the military Joint Trauma System Damage Control Resuscitation Clinical Practice Guideline (approved 10/2011) which currently recommends “The early use of TXA (ie, as soon as possible after injury but ideally not later than 3 hours postinjury) should be strongly considered for any patient requiring blood products in the treatment of combat-related hemorrhage and is most strongly advocated in patients judged likely to require massive transfusion (eg, significant injury and risk factors for massive transfusion)”.20 ,21
The European guidelines for management of bleeding and coagulopathy following major trauma (box 1) recommend early TXA administration in bleeding trauma patients, and “suggest that protocols for the management of bleeding patients consider administration of the first dose of TXA en route to the hospital. (Grade 2C)”.22 ,23 A review of TXA as part of damage control resuscitation in the prehospital setting concluded that “High-level evidence supports its use in trauma and strongly suggests that its implementation in the prehospital setting offers a survival advantage to many patients, particularly when evacuation to surgical care may be delayed”.24
Prehospital TXA recommendations from European guidelines (from Rossaint et al23)
Antifibrinolytic agents (Recommendation 25)
We recommend that tranexamic acid be administered as early as possible to the trauma patient who is bleeding or at risk of significant hemorrhage at a loading dose of 1 g infused over 10 min, followed by an intravenous infusion of 1 g over 8 hours. (Grade 1A)
We recommend that tranexamic acid be administered to the bleeding trauma patient within 3 hours after injury. (Grade 1B)
We suggest that protocols for the management of bleeding patients consider administration of the first dose of tranexamic acid en route to the hospital. (Grade 2C)
International Trauma Life Support (ITLS) recommends the following: “ITLS believes that there is sufficient evidence to support the use of TXA in the management of traumatic hemorrhage, pursuant to system medical control approval. Following initial resuscitation including control of external bleeding and stabilization of airway, consideration should be given to administration of TXA during early stages of transport. TXA should be considered in those patients who show signs of hemorrhagic shock, including tachycardia (>110 bpm) and hypotension (SBP<100) and are less than 3 hours from injury.”25
A recent ‘Guidance Document for the Prehospital Use of Tranexamic Acid in Injured Patients’ also made broad recommendations (box 2) endorsed by the American College of Surgeons Committee on Trauma, the American College of Emergency Physicians and the National Association of EMS Physicians.26 “Given the lack of data available, our organizations recommend that prehospital TXA administration be monitored closely in a prehospital and/or trauma registry. Administration should be reviewed and protocols constantly refined to avoid unnecessary or incomplete doses, inappropriate patient selection, or lack of infusion following the initial bolus. TXA dosing, timing, blood transfusion requirements, and any adverse events should be included in the registry”. Unfortunately, at present, there are no robust civilian data to guide which patients would potentially benefit from TXA administration in the prehospital setting.27
Prehospital TXA recommendations from US guidelines (from Fischer et al26)
TXA administration to bleeding patients
Objective measurements should be used to guide prehospital TXA administration protocols. The focus for management of compressible, external bleeding should be on direct pressure, tourniquets, hemostatic agents, and/or wound packing. Evidence of injury consistent with non-compressible hemorrhage (eg, penetrating thoracoabdominal trauma or unstable pelvis fractures) along with heart rate >120 bpm and SBP <90 mm Hg are suggested criteria. Agencies may consider vital sign adjustments for the geriatric population.
Don't forget the basics
In the bleeding patient, hemorrhage control and appropriate resuscitation remain the priority. Prehospital TXA use should never supersede field bleeding control techniques, rapid transport to a trauma center, or the administration of blood or plasma.
Ongoing pre-hospital TXA clinical trials
Two ongoing clinical trials are examining the efficacy of TXA in the prehospital setting for patients with severe injury and hemorrhage using exception from informed consent for emergency research.
STAAMP: study of TXA during air medical prehospital transport28,29
This multicenter trial is enrolling adult trauma patients being transported via air medical services from scene or referring to hospital, with SBP <90 mm Hg or heart rate >110 bpm and within 2 hours of injury. Patients will be randomized to a one-time prehospital bolus of TXA 1 g intravenous or placebo with three subsequent in-hospital groups (figure 4). The coordinating center is the University of Pittsburgh with multiple trauma center participating, and estimated total sample size 994 patients during a 3-year period of recruitment. Primary outcome measure is 30-day mortality. Predefined subgroups selected for additional exploratory analysis include subjects defined by (1) blood transfusion status; (2) traumatic brain injury; (3) transfer status; (4) requirement for operative intervention within 24 hours of admission; (5) therapeutic anticoagulation status; and (6) massive transfusion status. Two interim analyses are planned and will be overseen by the data safety monitoring board.
Two-phase STAAMP trial intervention schematic. From Brown et al.28
PATCH (Prehospital Antifibrinolytics for Traumatic Coagulatophy & Haemorrhage) study30,31
The PATCH-Trauma study is an international multicenter randomized, double-blind, placebo-controlled trial of prehospital TXA treatment for severely injured patients (target enrollment n=1184) at risk of acute traumatic coagulopathy. The study aims to determine the effects of early TXA administration on survival and recovery of severely injured patients treated within advanced trauma systems in Australia and New Zealand. The study is endorsed by the ANZICS Clinical Trials Group.32
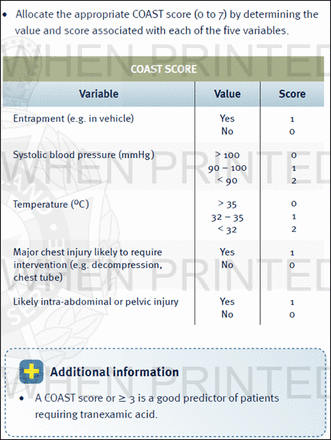
Adult trauma patients being transported to a trauma center with a prehospital coagulopathy of severe trauma (COAST) score of 3 or greater (figure 5), and within 3 hours of injury will be randomized to a prehospital bolus of TXA 1 g intravenous or placebo, followed by in-hospital TXA 1 g infusion over 8 hours in both groups. Primary outcome measures include mortality and functional recovery at 6 months. Secondary outcome measures include coagulation profiles, vascular events, ICU and hospital length of stay, and blood product use. Over 200 patients have been enrolled as of 9/2016.33 ,34
COAST (COAgulopathy in Severe Trauma) prehospital score used in PATCH clinical trial. From https://ambulance.qld.gov.au/%5Cdocs%5Cclinical%5Ccpp%5CCPP_COAST%20score.pdf.
Prehospital TXA for TBI35
An additional prehospital, multicenter, randomized, clinical trial aims to determine the efficacy of two prehospital TXA doses in moderate/severe TBI (GCS ≤12) compared to placebo: (1) 1 g TXA prehospital, 1 gm TXA infusion/8 hours; (2) 2 g TXA prehospital, placebo 0.9% NS/8 hours; (3) placebo 0.9% NS prehospital, placebo 0.9% NS/8 hours. The primary outcome measure is long-term neurological outcome measured by the Glasgow Outcome Scale Extended (GOS-E) score at 6 months postinjury.
The establishment of the Trans-Agency Consortium for Trauma-Induced Coagulopathy (TACTIC) funded by the NHLBI as a cooperative effort among NIH, the Department of Defense and other research centers will further investigate the clinical problem of trauma-induced coagulopathy and assist in making meaningful advances in this important field.36 ,37
Conclusion
Although we have made significant advances in the understanding of trauma-induced coagulopathy, there is still lack of clarity regarding links between diagnostic and laboratory coagulation testing and clinical bleeding risk.38 It is therefore evident that there is still significant controversy as to how best to manage trauma patients with severe injury and hemorrhage, including which patients would benefit most from TXA administration. At present, there is no definitive evidence to support efficacy of prehospital TXA administration in improving trauma outcomes. Data are lacking regarding which trauma patients might benefit, optimal dosing and timing and potential complications in the prehospital setting. Prehospital TXA protocols have not been adopted in most trauma centers. If prehospital TXA protocols are desired, issues to consider include time to definitive trauma care, feasibility of TXA intravenous administration, and how best to determine which patients would potentially benefit in the prehospital phase. The ongoing prehospital and in-hospital TXA randomized trials will provide additional high-quality evidence to support optimal clinical protocols for TXA use in the future. At present, the focus of prehospital care of the bleeding trauma victim should be hemorrhage control, hemostatic resuscitation and rapid transport to definitive hemorrhage control and definitive trauma care.
References
Footnotes
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.