Article Text
Abstract
Background Resuscitation for traumatic cardiac arrest (TCA) in patients with severe traumatic brain injury (sTBI) has historically been considered futile. There is little information on the characteristics and outcomes of these patients to guide intervention and prognosis. The purpose of the current study is to report the clinical characteristics, survival, and long-term neurological outcomes in patients who experienced TCA after sTBI and analyze the factors contributing to survival.
Methods A retrospective review identified 42 patients with TCA from a total of 402 patients with sTBI (Glasgow Coma Scale (GCS) score ≤8) who were admitted to Stony Brook University Hospital, a level I trauma center, from January 2011 to December 2018. Patient demographics, clinical characteristics, survival, and neurological functioning during hospitalization and at follow-up visits were collected.
Results Of the 42 patients, the average age was 45 years and 21.4% were female. Eight patients survived the injury (19.0%) to discharge and seven survived with good neurological function. Admission GCS score and bilateral pupil reactivity were found to be significant indicators of survival. The mean GCS score was 5.3 in survivors and 3.2 in non-survivors (p=0.020). Age, Injury Severity Score, or cardiac rhythm was not associated with survival. Frequent neuroimaging findings included subarachnoid hemorrhage, subdural hematoma, and diffuse axonal injury.
Discussion TCA after sTBI is survivable and seven out of eight patients in our study recovered with good neurological function. GCS score and pupil reactivity are the best indicators of survival. Our results suggest that due to the possibility of recovery, resuscitation and neurosurgical care should not be withheld from this patient population.
Level of evidence Level IV, therapeutic/care management.
- brain injuries
- traumatic
- glasgow coma scale
- heart arrest
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Traumatic brain injury (TBI) is a major cause of morbidity and mortality worldwide.1 Severe TBI (sTBI), defined as Glasgow Coma Scale (GCS) score equal or less than 8,2 often occurs in the setting of profound overall injury. One feared consequence of major trauma is traumatic cardiac arrest (TCA), which has a survival rate of less than 10% despite resuscitation by trained medical professionals.3 Because of the extremely poor prognosis, resuscitation of patients with TCA has been historically considered futile.3–5
The effect of cardiac arrest on the brain is well characterized. Much of the brain injury is not due to the initial insult, but rather to the inflammatory cascade that follows the initial cessation of cerebral blood blow.6 Persistent neurological deficits often result from secondary brain injury and neuronal cell death, which occur after the initial anoxic period.7 As a result, hypoxic-ischemic brain injury after cardiac arrest often results in long-term disability in survivors. However, reports of cardiac arrest after head trauma are extremely rare. Although studies of TCA from all trauma included some cases of TBI, the extent of their brain injury, neuroimaging characteristics, and more importantly long-term neurological function remain unclear.
Given complications that arise from cardiac arrest in addition to TBI, it is challenging to recommend neurosurgical interventions and provide prognostic information to families. Currently, it is thought that there is little chance of adequate recovery,3–5 but our study suggests that this may not necessarily be the case. The current study aims to characterize the clinical features, long-term survival and neurological outcomes, and prognostic factors for survival in patients who experienced cardiac arrest after sTBI. Our study would ultimately serve to provide information on management of patients with sTBI with cardiac arrest, who have been historically managed with extremely poor prognosis.
Patients and methods
Study design and data collection
We conducted a retrospective chart review at Stony Brook University Hospital, a level I trauma Center in Long Island, New York. Patient consent waivers were obtained from the Institutional Review Board office. We included all 402 adult patients (aged ≥18 years) during a period of 8 years (January 2011 and December 2018) who sustained sTBI, defined by a GCS score of 8 or less on presentation to the emergency department (ED). We then identified 42 patients who experienced TCA either outside of the hospital or at the ED. We excluded patients (1) with penetrating trauma, (2) who did not have TCA, (3) whose cardiac arrest happened after hospital admission or secondary to medical reasons (one patient had cardiac arrest during dialysis and subsequently fell), and (4) with significant cardiac comorbidities. We also excluded 11 patients who met the above criteria but whose care was withdrawn due to extremely poor functional outcome. All data were extracted from the hospital electronic medical record (Cerner Millennium, PowerChart).
Data were collected on age, sex, mechanism of injury, Injury Severity Score (ISS), GCS score on ED presentation, preintervention non-contrast head CT scans, initial pupil diameter and reactivity to light, neurosurgical interventions (external ventricular drain placement, hematoma evacuation, decompressive craniectomy), and length of stay. Regarding TCA, location (on the scene, transit, or at the ED), arrest rhythm, return of spontaneous circulation (ROSC), and airway management outside of the hospital (endotracheal intubation, bag mask ventilation, or laryngeal mask airway) were also recorded.
ISS was computed by summing the square of the three highest individual Abbreviated Injury Scale (AIS) scores. Head AIS score was listed separately. Non-contrast CT scan at admission was graded by the 6-point Rotterdam Scoring System.8 The Rotterdam scores evaluate injury severity based on the presence of basal cistern effacement, midline shift of >5 mm, epidural mass, and intraventricular or subarachnoid hemorrhage.
Outcomes
The primary outcome of the study is the survival status after TCA following sTBI. The surviving patients (n=8) were further evaluated at 6-month and 1-year postdischarge follow-up in the outpatient clinic. Data were obtained from clinical notes at these follow-up visits. Cases were scored according to Glasgow Outcome Scale-Extended and a modified Rankin Scale (mRS) for neurological disability at their follow-up appointments from 6 months to 1 year after discharge.9
Statistical analysis
Categorical variables were described as frequencies and percentages and were compared using χ2 test or Fisher’s exact test. Numerical variables were presented as mean with SD and compared using Student’s t-test. Variables with p values less than 0.05 were considered significant. All analyses were performed in SPSS V.26.
Results
Patient characteristics
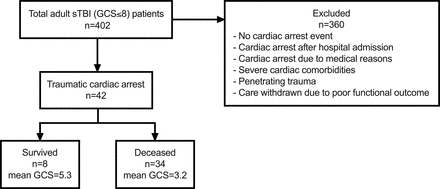
Of the 402 adult cases of sTBI at Stony Brook University Hospital from 2011 to 2018, 42 patients of TCA were included in the study, with 8 surviving patients at discharge (figure 1). As shown in table 1, of the 42 patients, the age range was 18 to 84 years, with a mean of 45 years. Of these patients 21.4% were female. The patients in this study suffered from severe head trauma with a mean GCS score of 3.5 and severe overall injury with a mean ISS of 38.2. The leading mechanism of injury was pedestrian struck (35.7%), followed by motor vehicle collision (28.6%), motorcycle crash (21.4%), and fall (14.3%).
Demographics and clinical characteristics of reviewed patients
Flow chart of patient selection. 42 patients with traumatic cardiac arrest (TCA) in the current study were selected from 402 patients with severe traumatic brain injury (sTBI) admitted to Stony Brook University Hospital from January 2011 to December 2018. Exclusion criteria were listed. The mean Glasgow Coma Scale (GCS) scores of the surviving and deceased patients with TCA were 5.3 and 3.2, respectively.
All 42 patients experienced cardiac arrest related to trauma, either prior to arriving at the hospital or very shortly after presentation to the ED. Figure 2 illustrates the cardiac rhythms before cardiopulmonary resuscitation (CPR). The most common rhythm was asystole (38.1%), followed by pulseless electrical activity (PEA) (35.7%) and ventricular arrhythmia (4.8%). Regarding emergent airway management, 30 (71.4%) patients were intubated at the scene of injury and two (4.8%) received bag mask ventilation.
Cardiac rhythms before initiation of cardiopulmonary resuscitation. The most common cardiac rhythm is asystole (38.1%), followed by pulseless electrical activity (PEA) (35.7%).
The survival rate in patients who experienced TCA after sTBI was 19.0% (8 out of 42). In the 34 patients who did not survive, the most common cause of death was cardiac arrest in 27 patients (64.3%), followed by brain death in 7 (16.7%).
Characteristics of survivors
Of the total 42 patients with TCA in this study, eight survived to discharge (table 2). The mean age of the survivors was 38.6 years (range, 18–66), with two women and six men. The average length of stay was 53.3 days (range, 9–100). Three (37.5%) of these patients were pedestrians struck at high speeds, three (37.5%) were in motor vehicle crash, and two were motorcyclists struck by cars (25%). These cases all had additional injuries apart from sTBI, demonstrated by a mean ISS of 33.6. Two patients achieved ROSC under 5 minutes. All eight patients had no known cardiac comorbidities prior to sTBI presentation. All survivors followed commands during their hospitalization. The average time to command following was 16.1 days (range 2–36 days).
Clinical characteristics of survivors
Seven out of eight patients survived with good neurological function, as demonstrated by an mRS score of equal or less than 4 at their follow-up visits. At 6-month to 1-year follow-up, two patients had good recovery with an mRS score of 1. They were able to carry out activities of daily living independently, although with residual symptoms (right eye vision loss in patient 5). Two patients had slight disability but were able to carry out daily activities with minimal assistance. Three patients had moderate to severe disability (paraplegia) but had the ability to communicate and/or follow commands. One patient scored 5 on the mRS, requiring constant nursing care, as well as a tracheotomy and a gastrostomy.
Factors contributing to survival
We compared the clinical and imaging characteristics of survivors and non-survivors (table 3). Survivors had higher overall GCS score (p=0.020) and its motor component (p=0.026) compared with non-survivors. Pupil reactivity on ED presentation was significantly different between survivors and non-survivors (p=0.001) (figure 3). The majority of non-survivors had non-reactive and dilated pupils, although three of them had bilateral reactivity on initial presentation. This was in contrast to survivors, of whom the majority had bilateral pupil reactivity, with only two who presented with non-reactive and constricted pupils. Patients who presented with bilateral reactivity at admission had a survival rate of 62.5%, whereas those presenting with non-reactive pupils had a survival rate of only 8.8%. There was no significant difference in age, gender, ISS, or mechanisms of injury between survivors and non-survivors. Similarly, the location of CPR initiation, intubation status prior to hospital arrival, or traumatic arrest rhythm did not contribute significantly to mortality.
Comparisons between survivors and non-survivors of traumatic arrest after TBI
Pupil diameter and reactivity in patients with traumatic brain injury with traumatic cardiac arrest are compared between survivors and non-survivors in four categories.
Neuroimaging and neurosurgical interventions
Non-contrast head CT was performed in 26 out of 42 patients with TCA. The rest died soon after ED arrival, before they could be scanned. Of the patients 21 (80.7%) showed evidence of subarachnoid hemorrhage, 11 (42.3%) had subdural hematoma, and 8 (30.8%) showed diffuse hypoxic injury with poor gray-white matter differentiation. Seven patients had evidence of herniation on neuroimaging, including transtentorial, subfalcine, and tonsillar herniation. The mean Rotterdam score was 2.8.
Interestingly, 87.5% of the survivors received intracranial pressure monitoring, significantly more than 11.8% in the non-survivors (p<0.001). This was likely due to patients who did not survive dying rapidly from the injuries, neurosurgery interventions being considered futile, or risks outweighing the benefits. Of the patients 16.7% underwent neurosurgical procedures. Seven patients received external ventricular drainage and two underwent decompressive craniectomy.
Discussion
Resuscitation of patients with TCA has a low success rate and is traditionally considered futile.3–5 Little is known about patients with TCA after sTBI, which is a major cause of death and disability in itself. Our study demonstrates that, despite the grim odds of survival and extensive injury, some patients do survive, even with good neurological function. Although limited by the small sample size, the current study has a survival rate of 19.0%, which is comparable with cardiac arrest associated with all other trauma, and even as good or better than outcomes from out-of-hospital cardiac arrest of any cause.10 11 It is important to note that some of our cases of survival achieved ROSC less than 5 minutes, which may contribute to the positive neurological outcomes observed. Our results show that TBI may not substantially affect TCA survival, and timely resuscitation can yield good neurological outcomes.
The two most important clinical features that provide prognostic information in our study are GCS score and pupil reactivity. GCS is routinely used to evaluate neurological function in patients who have sustained head injuries. We showed that GCS score is a significant predictor of survival after cardiac arrest associated with sTBI. GCS motor score is also significantly higher in survivors compared with non-survivors. This result is consistent with previous studies,12 13 including the CRASH trial,14 which demonstrated strong correlation between admission GCS score with mortality and morbidity at discharge and at 6-month follow-up. The GCS score in this study is recorded on arrival at the ED, which has been shown to have higher predictive value than those obtained on the scene as a result of initial management of airway, breathing, and circulation.13 On the contrary, ISS was not a significant predictor in our patient population. This is partly because all of our patients have very severe overall injury (ISS >15). The results of our study suggest that neurological function assessment by GCS outweighs overall injury in providing prognostic information in sTBI.
Pupil diameter and reactivity to light provide vital information on the extent of brain damage. A dilated unreactive pupil indicates compression of the oculomotor nerve, often due to midline shift and uncal herniation, and thus worse prognosis. This was reflected in our results that most of the patients who survived had bilaterally reactive pupils, whereas almost all of those with non-reactive and dilated pupils did not survive. Previous report has also shown that pupillary reactivity is a reliable prognostic indicator of mortality in sTBI both on the field and at admission, compared with GCS, making it a more useful early indicator of survival.15 One limitation of pupil reactivity is that it could be affected by sedative medications. However, most of our patients only had light sedation on arrival to the ED. It is important to note that previous studies have made the effort to combine GCS score and pupil response to provide more accurate prognosis of patients with sTBI, highlighting the importance of both clinical assessments in evaluating severe brain trauma.8 Our study is in concordance with these findings in that GCS score and pupil response are the single two most important indicators of survival.
The most common initial rhythm before cardiac arrest in our study is PEA, followed by asystole. These two cardiac rhythms are most commonly associated with obstructive, hypovolemic, and cardiogenic shock as well as tissue hypoxia.16 17 Although closely related to traumatic injury, no specific cardiac rhythm was significantly associated with mortality in our study. Other general clinical characteristics related to trauma, such as mechanisms of injury, intubation status on the scene, and the location of cardiac arrest, also did not significantly contribute to mortality in our cohort, which is consistent with current literature on TCA with larger sample sizes.10 16 18 Studies focusing on the relationship between age and mortality from TCA have mixed findings. Majority shows that older age is associated with worse outcome.11 19 In our study, however, there was no difference in age between patients who survived and those who did not. This result could be attributed to the fact that we excluded patients with severe chronic comorbidities and cardiac arrest due to medical causes. Both of these conditions have increased incidence with age and forebode worse outcomes seen in elderly patients.20 21
Several consequences of brain trauma after TCA are evidenced on imaging. In our study, most patients who underwent CT scans had radiological evidence of subarachnoid hemorrhage and subdural hematoma. Other frequent neuroimaging findings in patients include diffuse axonal injury with poor gray-white matter differentiation. Hypoxic-ischemic brain injury is one of the most serious consequences of cardiac arrest, reported to be present in one-fifth of all out-of-hospital cardiac arrest cases.6 22 The effects of cardiac arrest on brain tissue are twofold—a primary injury immediately as a result of cessation of cerebral blood flow and a secondary injury related to reperfusion, which takes hours or days to occur. Whether the brain imaging findings in our study are attributed to the primary or the secondary mechanisms is not clear. Previous mechanistic studies have found common biomarkers of cardiac arrest and TBI, which may suggest shared pathophysiological cascades in these two insults.23 Future studies are needed to elucidate the biochemical changes in the brain tissue in cases of TCA after sTBI.
Our study is a retrospective chart review and is subject to limitations of the study design. The sample size is small, partially limited by the rare event of cardiac arrest after sTBI. We recognize that GCS score at admission may be affected by confounding effects from sedation, neuromuscular blockade, and rapid sequence intubation during resuscitation on the field, as well as inter-rater discrepancy. Nevertheless, GCS remains an essential clinical tool for evaluating the level of consciousness when patients with severe trauma arrive at the hospital. We did not include the underlying health status of the patients in the analysis, which could be a factor contributing to their survival and recovery. However, we did exclude those with significant cardiac comorbidity and those with cardiac arrest due to other medical reasons. Limited by the nature of chart review study, we did not analyze the transport time from cardiac arrest to ED arrival, CPR duration, or time to definitive airway, which could also impact the success of resuscitation. Nonetheless, we think that the reported data are representative of the TCA population in general.
In conclusion, this study demonstrates that patients with cardiac arrest after sTBI do survive and can recover with decent neurological function, although the mortality rate remains high. Initial clinical features including GCS score, pupillary diameter, and reactivity to light were important indicators of survival. Some frequent neuroimaging findings on non-contrast head CT in this unique group of patients include subarachnoid hemorrhage, subdural hematoma, and hypoxic axonal injury. Given the chance of survival and neurological recovery, cardiac arrest event should not exclude patients with sTBI from getting proper resuscitation and appropriate neurosurgical care.
Acknowledgments
We would like to thank Jane McCormack, Emily Huang, and the Stony Brook Trauma, Emergency Surgery, and Surgical Critical Care Division faculty and staff for their assistance in this study.
References
Footnotes
Contributors Conception and design: CBM, ZZ, JJL, SM. Acquisition of data: CBM, ZZ, JJL, NJW, MM, SD, AF. Analysis and interpretation of data: CBM, ZZ, JJL, ZW, NJW, MM, SD, AF, SMF, SM. Drafting the article: CBM, ZZ, JJL, SM. Critically revising the article: CBM, ZZ, JJL, ZW, SM. Approved the final version of the article on behalf of all authors: CBM. Statistical analysis: CBM, ZZ, JJL. Administrative/technical/material support: CBM, ZZ, JJL. Study supervision: CBM, SM.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval The study was approved by the Stony Brook Institutional Review Board (IRB-2019-00464).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request. The data set used in this manuscript is part of a larger traumatic brain injury database at our institution. We currently do not have the approval from IRB to share any portion of the data set as it is protected patient information. Please contact the corresponding author for data set requests at this time.